Progress Note 2024: Curing HIV; Not in My Lifetime or Just Around the Corner?
Justin Harper1, Michael R. Betts2,3, Mathias Lichterfeld4,5, Michaela Müller-Trutwin6, David Margolis7, Katharine J. Bar3,8, Jonathan Z. Li9, Joseph M. McCune10, Sharon R. Lewin11,12,13, Deanna Kulpa1,14, Santiago Ávila-Ríos15, Dázon Dixon Diallo16, Michael M. Lederman17, Mirko Paiardini1,14
1Division of Microbiology and Immunology, Emory National Primate Research Center, Emory University, Atlanta, Georgia
2Department of Microbiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania
3Center for AIDS Research, University of Pennsylvania, Philadelphia, Pennsylvania
4Ragon Institute of MGH, MIT and Harvard, Cambridge, Massachusetts
5Infectious Disease Division, Brigham and Women’s Hospital, Boston, Massachusetts
6HIV Inflammation and Persistence Unit, Institut Pasteur, Université Paris-Cité, Paris, France
7Division of Infectious Diseases, Center for AIDS Research, University of North Carolina at Chapel Hill, School of Medicine, Chapel Hill, North Carolina
8Department of Medicine, Perelman School of Medicine, University of Pennsylvania,
Philadelphia, Pennsylvania
9Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
10HIV Frontiers, Global Health Accelerator, Bill & Melinda Gates Foundation
11Department of Infectious Diseases, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
12Victorian Infectious Diseases Service, Royal Melbourne Hospital at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
13Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Australia
14Department of Pathology and Laboratory Medicine, Emory University, Atlanta, Georgia
15Centro de Investigación en Enfermedades Infecciosas, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico
16SisterLove, Inc., Atlanta, Georgia
17Division of Infectious Diseases and HIV Medicine, Case Western Reserve University, Cleveland,
Ohio
Michael M. Lederman, lederman.michael@clevelandactu.org
Mirko Paiardini, mirko.paiardini@emory.edu
Harper J, Betts MR, Lichterfeld M, Müller-Trutwin M, Margolis D, Bar KJ, Li JZ, McCune JM, Lewin SR, Kulpa D, Ávila-Ríos S, Diallo DD, Lederman MM, Paiardini M. Erratum to: Progress Note 2024: Curing HIV; Not in My Lifetime or Just Around the Corner? Pathogens and Immunity. 2024;8(2):115-157,179. doi: 10.20411/pai.v8i2.665
10.20411/pai.v8i2.665
Once a death sentence, HIV is now considered a manageable chronic disease due to the development of antiretroviral therapy (ART) regimens with minimal toxicity and a high barrier for genetic resistance. While highly effective in arresting AIDS progression and rendering the virus untransmissible in people living with HIV (PLWH) with undetectable viremia (U=U) [1, 2], ART alone is incapable of eradicating the “reservoir” of resting, latently infected CD4+ T cells from which virus recrudesces upon treatment cessation. As of 2022 estimates, there are 39 million PLWH, of whom 86% are aware of their status and 76% are receiving ART [3]. As of 2017, ART-treated PLWH exhibit near normalized life expectancies without adjustment for socioeconomic differences [4]. Furthermore, there is a global deceleration in the rate of new infections [3] driven by expanded access to pre-exposure prophylaxis (PrEP), HIV testing in vulnerable populations, and by ART treatment [5]. Therefore, despite outstanding issues pertaining to cost and access in developing countries, there is strong enthusiasm that aggressive testing, treatment, and effective viral suppression may be able to halt the ongoing HIV epidemic (ie, UNAIDS’ 95-95-95 targets) [6–8]; especially as evidenced by recent encouraging observations in Sydney [9].
Despite these promising efforts to limit further viral transmission, for PLWH, a “cure” remains elusive; whether it be to completely eradicate the viral reservoir (ie, cure) or to induce long-term viral remission in the absence of ART (ie, control; Figure 1). In a previous salon hosted by Pathogens and Immunity in 2016 [10], some researchers were optimistic that a cure was a feasible, scalable goal, albeit with no clear consensus on the best route. So, how are these cure strategies panning out? In this commentary, 8 years later, we will provide a brief overview on recent advances and failures towards identifying determinants of viral persistence and developing a scalable cure for HIV. Based on these observations, and as in the earlier salon, we have asked several prominent HIV cure researchers for their perspectives.
HIV; SIV; reservoir; persistence; ART; HIV cure; HIV control

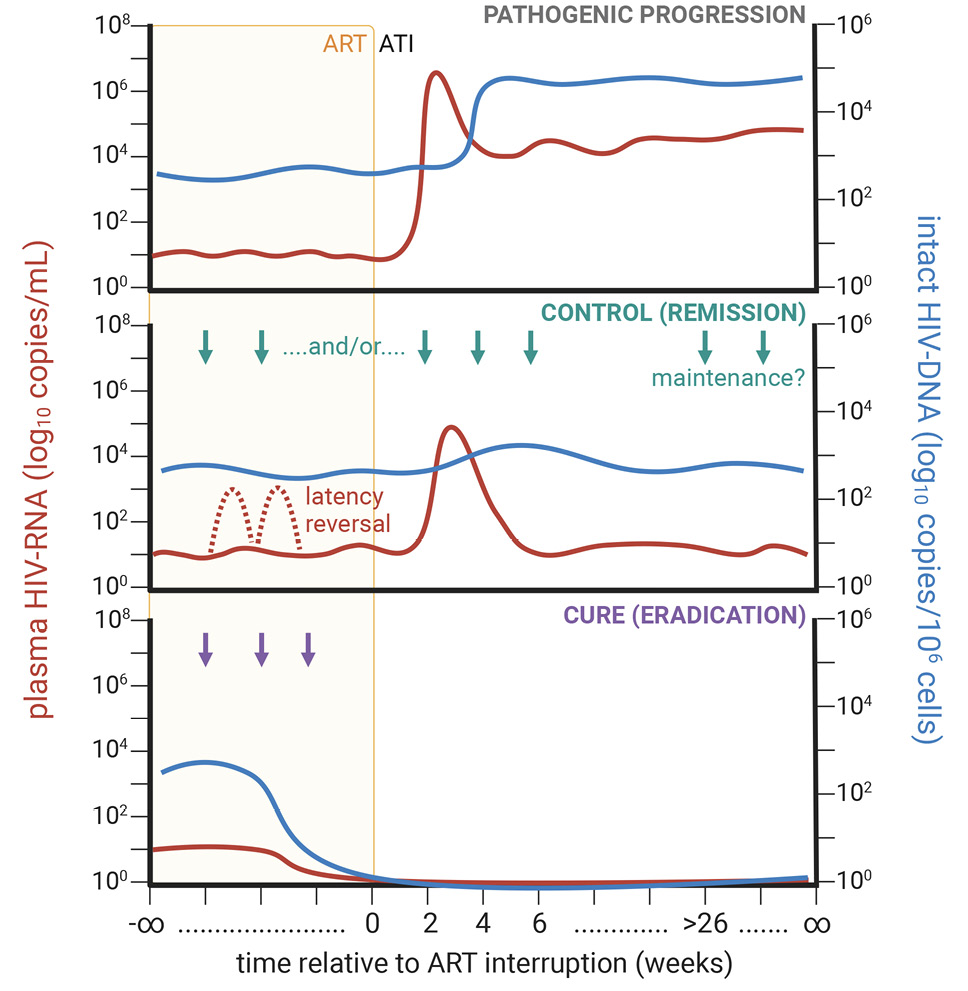
Figure 1. Conceptualization of longitudinal HIV-1 plasma RNA (at left; red line) and tissue DNA content (at right; blue line) during long-term antiretroviral therapy (ART; orange background) and following ART analytical therapy interruption (ATI; white background) for pathogenic disease progression (top; grey) and therapies conferring viral control (middle; green), including potentially with latency reversal (dashed line), or cure (bottom; purple).
While antiretroviral therapy (ART) suppresses plasma viremia to clinically undetectable levels and blocks de novo cellular infection, the reservoir of latently infected cells is remarkably stable during ART [11], thereby facilitating the rapid rebound of systemic viremia upon analytical treatment interruption (ATI) [12, 13]. The latent reservoir is largely formed by the infection of CD4+ T cells transitioning from an effector to resting memory state [14] and is established very early on during acute infection as independent events [15–17] with sequences of replication-competent virus during long-term ART reflecting those detected at ART initiation [18]. Early ART initiation is beneficial in limiting viral transmission [19], preserving immune function [20], reducing the size of the viral reservoir [21], and lessening inflammation [22], and as an experimental model to test interventions aimed at disrupting the establishment of viral latency [23]. Outside of effective use as post-exposure prophylaxis (PEP) [16, 24], the mass implementation of very early ART as a cure strategy is doubtful, as the reservoir is established prior to the detection of systemic viral RNA [15, 25, 26] (ie, Fiebig stage I) [27].
After undergoing an initial contraction with ART, the viral reservoir expands [28] via clonal expansion by homeostatic [29], antigen-induced [30–32], and possibly integration site-linked proliferation [33–35], which is supported by the survival of quiescent, long-lived cellular viral reservoirs, such as stem cell memory (TSCM) CD4+ T cells [36, 37]. Chromosomal integration site analyses have demonstrated selection against intact provirus with transcriptional activity during long-term ART [38, 39], which, while occasionally violated by large clones [40], is indicative of host immune surveillance. Moreover, these transcriptionally active viral reservoirs [41, 42], while incapable of de novo reseeding amid effective ART, promote the maintenance of HIV-specific CD4+ and CD8+ T cells [43, 44] and induce a state of functional anergy and impaired differentiation (ie, exhaustion) due to chronic antigenic stimulation. As these latent reservoirs persist for life, much work has been done to comparatively identify determinants of viral control and immune homeostasis in elite controllers (ie, people living with HIV (PLWH) who maintain undetectable viremia in the absence of ART), post-treatment controllers (PTCs; ie, PLWH who control viremia following ATI), and nonhuman primate (NHP) natural hosts (ie, NHPs that do not progress to AIDS despite high levels of viral replication). While non exhaustive (Table 1), many key determinants of viral control and persistence have been identified encompassing an array of mechanisms. It remains to be determined which of these proposed mechanisms is the most essential to facilitate viral control or if they can be simultaneously targeted without toxicity.
The cellular tropism of HIV is primarily CD4+ T cells, and to a lesser extent macrophages [68], based on their expression of CD4, the primary receptor for viral entry, and the CCR5 and CXCR4 co-receptors, which identify the virus as R5 or X4 tropic, respectively; however, selective biomarkers for identifying infected cells remain elusive [69–72]. The use of microfluidic and single-cell sequencing approaches has enabled the isolation of unstimulated blood and lymph node CD4+ T cells harboring HIV-DNA, including those with only intact provirus, from PLWH on long-term ART. These analyses reveal that infected cells exhibit transcriptomic signatures favoring HIV silencing, cell survival, and proliferation [73], and clusters of surface receptors associated with immune checkpoint signaling, cell survival, and resistance to cytotoxic killing [74]. Yet, no individual marker, including CCR5, selectively discriminated CD4+ T cells harboring HIV-DNA. Likewise, the antibody-mediated depletion of the entire CD4+ T cell compartment during ART reduced the absolute numbers, but not the frequency, of infected cells and failed to limit viral recrudescence, with or without reconstitution of CD4+ T cells prior to ATI [75, 76]. Therefore, it is likely the only currently feasible route to selectively target infected cells is by their expression of viral envelope (Env) proteins on their cell surface, thereby rendering latently infected cells impervious to detection during ART barring the stimulation of viral replication by latency reversing agents (LRAs).
Table 1
|
Determinant or Correlates of Viral Controla |
Potential Targetsb |
Cohortc |
Speciesd |
|
robust, but transient, type-I interferon response [45] |
IFNα |
natural host |
AGM |
|
low inducible CCR5 expression on CD4+ TCM [46] |
CCR5 |
natural host |
SM |
|
migration of NK cells into the lymphoid B cell follicle [47] |
CXCR5 |
natural host |
AGM |
|
blunted LPS responsiveness and reduced cell adhesion [48] |
TLR4, ICAM-2 |
natural host |
SM |
|
increased NK cell terminal differentiation [49] |
NKG2a |
natural host |
AGM |
|
homing of CD8 T cells to lymphoid B cell follicle [50] |
CXCR5 |
EC |
RM |
|
reduced dysbiosis of the gut microbiota [51] |
microbiome |
EC |
Human |
|
metabolic plasticity of HIV-specific CD8+ TCM [52] |
IL-15 |
EC |
Human |
|
differentiation, polyfunctionality of HIV-specific CD8+ T cells [53] |
TOX, TCF-1 |
EC |
Human |
|
viral integration in heterochromatin regions [54] |
|
EC |
Human |
|
immune selection on intact proviruses [55] |
Nef |
EC |
Human |
|
stemness, survival, and plasticity of HIV-specific CD8+ T cells [56] |
WNT, TCF-1, mTORC |
EC |
Human |
|
low reservoir content in CD4+ TN and TCM [57] |
early ART |
PTC |
Human |
|
low reservoir content during ART [58] |
|
PTC |
Human |
|
low reservoir content and gut Th17 cell homeostasis [59] |
|
PTC |
RM |
|
Env-specific memory B cell responses [60] |
|
PTC |
Human |
|
low reservoir content, dominated by CD4+ TTM and TEM [61] |
early ART |
PTC |
Human |
|
Determinant or Correlates of Viral Persistence |
Potential Targets |
Status |
Species |
|
PD-1, CTLA-4 |
pathogenic |
Human |
|
|
viral persistence in lymphoid CD4+ TFH [64] |
PD-1 |
pathogenic |
Human |
|
resistance to cytolytic killing [65] |
BCL-2 |
pathogenic |
Human |
|
IL-10 related CD4+ T cell survival [66] |
IL-10, STAT3 |
pathogenic |
RM |
|
CD8+ T cell-mediated non-cytolytic control of latency [67] |
WNT, TGF-β, β-catenin |
pathogenic |
Human |
aProposed mechanism of viral control status: central memory T cell (TCM), lipopolysaccharide (LPS), natural killer (NK), naïve T cells (TN), T helper 17 (Th17), transitional memory T cells (TTM), effector memory T cells (TEM), follicular T helper cell (TFH).
bReceptors or signaling cascades regulating viral control status, if identified.
cType of viral control status: elite controller (EC), post-treatment controller (PTC).
dSpecies of cohort: African green monkeys (AGMs), rhesus macaques (RM).
Based on these considerable barriers enabling viral persistence during ART, cure strategies have been developed to reverse viral latency, thereby rendering infected cells susceptible to immune clearance (ie, “shock and kill”; Figure 2) [77]. While numerous immunotherapies have been validated as LRAs in vivo, these approaches failed to substantially reduce the size of the viral reservoir and demonstrated very limited efficacy at the maximum tolerable dose in PLWH (eg, histone deacetylase (HDAC) inhibition [78] and IL-15 superagonists [79]). While initially promising in pre-clinical models, many LRAs have thus far failed to progress to clinical trials due to safety concerns (eg, CD8 depletion [80], combination immune checkpoint blockade [81, 82], and the inhibition of noncanonical NF-κB signaling via administration of second mitochondria-derived activator of caspase, SMAC, mimetic [83, 84]) or have shown inconsistent responses (eg, TLR7 agonists) [85–87]. Recently, the administration of recombinant IL-2 (Aldesleukin) to ART-suppressed PLWH (n=9) induced a 26-fold increase in plasma viremia 3 days after a 4-day cycle (NCT03308786); however, toxicities resulted in interruption of the trial. Insofar as some latently infected cells exhibit a high barrier to expression [88, 89], it is unlikely currently utilized LRAs will be capable of inducing viral production from all replication-competent proviruses, a major limitation of the “shock and kill” approach, as a rare replication-competent virion can initiate systemic viral recrudescence [90]. Consistent with this constraint, LRA-mediated reductions in viral burden during ART are insufficient to affect viral rebound kinetics upon treatment interruption [81]. Therefore, in the absence of durable immune-mediated viral control, it is unlikely the “shock and kill” approach alone is a viable pathway towards an eradicative cure. Alternatively, given the low inducibility of some integrated viruses, an opposing approach under investigation is to induce potent epigenetic silencing to prevent viral recrudescence in the absence of ART (“block and lock”) [91].
Other immunotherapy-based strategies currently under investigation include disrupting the establishment of viral latency in acute infection and/or at ART initiation and intercepting viral recrudescence following ATI to establish durable immune control. For example, PD-1 blockade in combination with IL-10 neutralization at ATI suppresses viral replication post rebound, albeit with variable responsiveness and significant toxicity [92]. Although the exact mechanisms are still to be determined, this study provides a proof-of-concept that those pathways can be harnessed to tilt the balance towards immune control of a highly replicative virus, such as SIVmac239. Given the absence of effective vaccines [93–96] or therapies to elicit durable and potent HIV-specific T cell responses able to control the rebounding virus, next-generation broadly neutralizing antibodies (bNAbs) directed against the viral envelope have emerged as a leading candidate to eliminate cells supporting viral replication via natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC; Figure 2). When administered during ART in combination with LRAs, bNAb therapy permits the targeted labeling and clearance of infected cells supporting viral reactivation [84]; however, the same limitations regarding incomplete induction outlined with “shock and kill” strategies still apply. When given at ATI or during chronic infection, bNAbs are efficacious in suppressing, but not preventing, viral rebound upon either treatment cessation or viral escape amid ongoing therapy [97–103]. While combination bNAb regimens with broad epitope coverage have proven effective in limiting viral escape [104–108], there is considerable interest in identifying if there is complementarity between bNAb-targeted epitopes, such that escape from one bNAb enhances sensitivity to others (eg, inverse resistance pathways), a phenomenon previously observed with specific ART regimens [109]. It has been suggested that bNAbs may also exert a vaccinal effect to support HIV-specific T cell immunity [110, 111], although this is a matter of extensive debate in the field. Irrespective, bNAbs alone are insufficient to purge the pool of latently infected cells, and further studies are needed to assess synergy with other treatment modalities towards controlling HIV in the absence of ART.
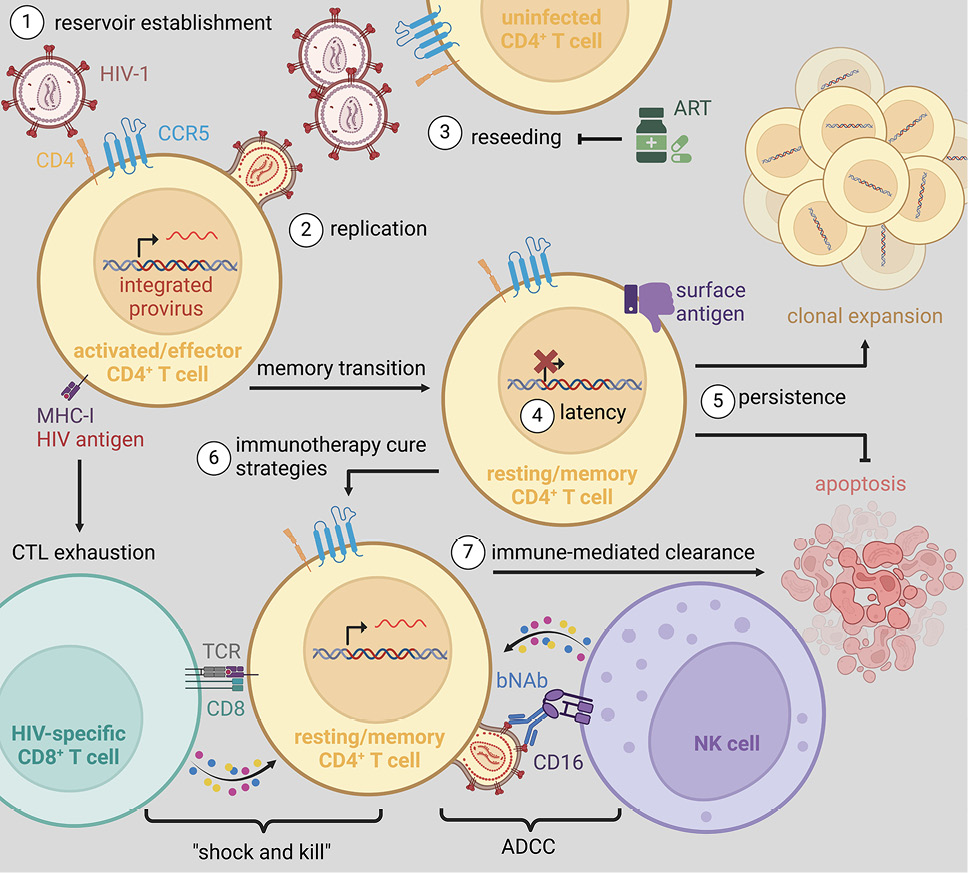
Figure 2. (1) CCR5+ CD4+ T cells are efficiently infected with HIV-1 resulting in the establishment of a pool of productively infected cells. (2) These highly activated effector CD4+ T cells support robust levels of viral replication facilitating the rapid escape from cytolytic T lymphocyte (CTL) responses and inducing a state of CTL exhaustion due to chronic antigenic stimulation. (3) Viral replication leads to the rapid, exponential infection of bystander CD4+ T cells and systemic viremia that progressively contributes to CD4+ T cell depletion and AIDS progression. Alternatively, antiretroviral therapy (ART) is highly effective in blocking de novo infection of vulnerable cells, thereby indirectly reducing plasma viremia as productively infected cells turn over due to viral cytopathic effects and/or immune-mediated clearance. (4) A subset of productively infected effector CD4+ T cell reverts to long-lived, resting, memory cells in which viral latency is established. (5) During long-term ART, latently infected memory CD4+ T cells persist indefinitely. This persistence has been linked to clonal expansion via homeostatic proliferation, inhibition of apoptotic pathways (ie, pro-survival), and impaired immunosurveillance. (6) Some immunotherapy cure strategies seek to transiently reverse viral latency to enhance viral peptide presentation and the expression of viral envelope on the cell surface (ie, “shock”), thus rendering latently infected cells susceptible to elimination by HIV-specific CD8+ T cells or by natural killer (NK) cell mediated antibody-dependent cellular cytotoxicity (ADCC; ie, “kill”), respectively. (7) To promote the immune-mediated clearance of reactivated cells, in an environment where ART protects uninfected cells from infection, combination therapies may be applied to augment the cytotoxicity, activation, homing, and/or differentiation of responding CD8+ T cells and NK cells.
Despite the complexity of the mechanisms related to viral persistence and the shortcomings with immunotherapy-based interventions, there is evidence that a cure is theoretically possible. For example, long-term viral control following ATI has been observed in 5 patients who underwent allogeneic hematopoietic stem cell transplantation (alloHSCT) using cells from donors homozygous for the CCR5D32 mutation (CCR5D32/D32) [112] to prevent subsequent viral infection of engrafted CD4+ T cells upon attaining complete chimerism (Figure 3): ie, the Berlin [113, 114], London [115, 116], New York [117], Düsseldorf [118], and City of Hope [119] patients. These patients have been defined as cured, and their experience serves as a proof-of-concept that cure is possible. As these are case studies, efficacy is likely highly skewed due to publication bias for positive results, as there are limited reports of patient deaths [120–123] as would be anticipated with alloHSCT. It is also plausible that, in a subset of patients, the CCR5D32/D32 mutation could select for X4 tropic virus resulting in viral escape [124] as observed with CCR5 inhibitors in the absence of suppressive ART [125]. As currently implemented, this strategy is only advisable for management of aggressive leukemias and lymphomas as it requires pre-conditioning regimens with significant toxicity, including total body irradiation, chemotherapy, and immunomodulatory therapies, and entails a substantial risk of graft-versus-host disease (GvHD). It should be noted that myeloablative conditioning in and of itself is insufficient to promote viral control [126–128]. Additionally, alloHSCT is poorly scalable as it requires identification of HLA-matched donors possessing the rare CCR5D32/D32 mutation [129, 130].
To overcome these limitations, gene editing platforms are being adapted to develop “off-the-shelf” products to disrupt CCR5. For example, in situ CRISPR editing to disrupt CCR5 [131] or proviral sequences [132] with non-integrating viral delivery vectors have shown promise in facilitating viral reduction in preclinical animal models. The ex vivo disruption of CCR5 in blood CD4+ T cells using zinc-finger nucleases (ZFNs) [133] accords significant resistance to HIV in vitro [134]; yet, autologous infusions of these cells to ART-treated PLWH results in a limited impact on the kinetics of viral rebound with ATI or the size of the viral reservoir following ART re-initiation [135, 136]. Poor efficacy is tentatively linked to the extent of cell engraftment, which may be ameliorated by differentiating cells towards a stem cell memory phenotype prior to infusion [137] or by additional selection mechanisms to enhance host CD4<sup>+</sup> T cell depletion, such as short periods of ART interruption. A competing hypothesis is that viral elimination in these patients is dependent on allogeneic immune responses, as all but one of the cured CCR5D32/D32 alloHSCT patients exhibited GvHD (Figure 3) [117]. In this regard, limited viral control has been observed in patients with GvHD undergoing alloHSCT with donor cells that are heterozygous (CCR5D32/WT; Boston, rebounded at 84 and 225 days [90, 138]) or wildtype (CCR5WT/WT) for the CCR5 alleles (Minnesota, rebounded at 288 days [139]; Geneva, undetectable as of 20-months of follow-up [140]). In SIV-infected macaques, the formation of GvHD following CCR5WT/WT alloHSCT was proven to be essential in promoting the stepwise clearance of the viral reservoir across tissue compartments resulting in long-term viral remission [141], analogous to graft-versus-leukemia (GvL) elimination of malignant cells [142, 143]. While providing compelling support for the role of allogeneic immunity, these data suggest that GvHD may be a necessary feature for those cure strategies and that alternative strategies trying to avoid GvHD, such as via gene-edited autoHSCT, may have a more limited impact on eliminating the reservoir. This raises significant concerns given GvHD’s complicated morbidity, clinical management [144], and mortality rate, that may further limit scalability.
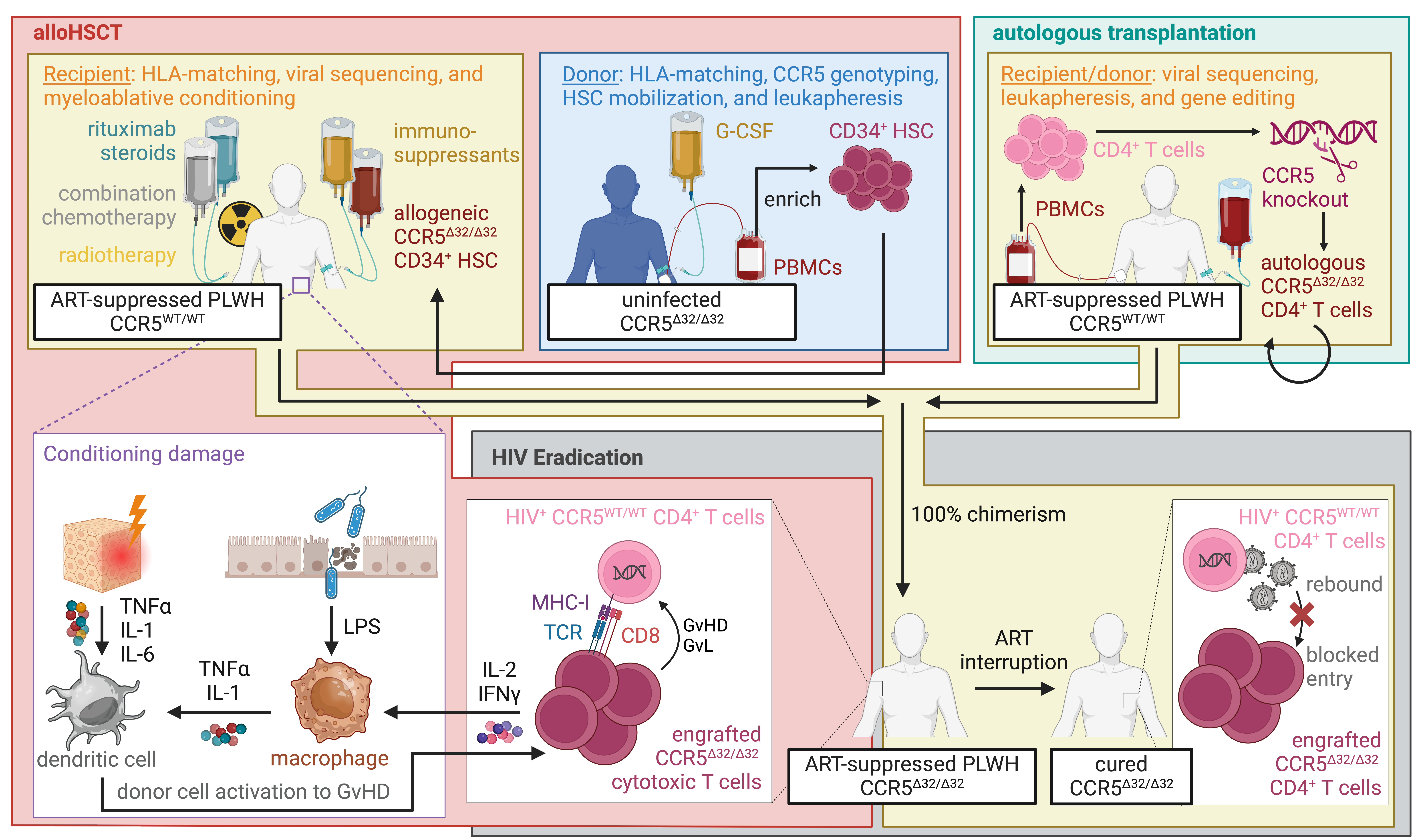
Figure 3. Schematic of the workflow for hematopoietic stem cell transplantation (HSCT) to an ART-suppressed PLWH with wild-type CCR5 (yellow background). Allogeneic HSCT (alloHSCT; red background) is performed using mobilized CD34+ HSCs isolated from the blood of an uninfected CCR5D32/D32 donor (blue background) and administered to the patient following conditioning with chemotherapy, radiotherapy, immunosuppressants, and/or immunomodulators to promote cell engraftment and to prevent graft rejection. The alloHSCT conditioning regimen induces systemic tissue damage and a breakdown of the gut epithelium resulting in the production of pro-inflammatory cytokines (IL-1, IL-6, and TNFa) and the influx of bacterial elements, such as lipopolysaccharides (LPS), causing robust dendritic cell and macrophage activation. Autologous transplantation (green background) is performed using CD4+ T cells isolated from the blood of the ART-suppressed PLWH. Cells for autologous transplantation then undergo ex vivo gene editing to knock out CCR5 and are then re-infused to the donor. Eradication of the HIV reservoir (grey background) occurs upon complete chimerism with donor cells. Infected CD4+ T cells are subject to clearance by activated donor cytotoxic T cells against minor recipient antigens as a graft-versus-host disease (GvHD) response. Upon ART interruption, engrafted donor CCR5D32/D32 CD4+ T cells are refractory to infection if virus is produced by a residual reservoir.
Stunning progress has been made in the development of antiretrovirals with reduced toxicity and/or high barriers for genetic resistance. This progress includes the development of non-boosted integrase inhibitors (dolutegravir, DTG [145–147]), reduced dosage prodrugs (tenofovir alafenamide, TAF [148, 149]), nucleoside reverse transcriptase translocation inhibitors (NRTTIs; islatravir [150–152]), capsid assembly inhibitors targeting multiple steps of viral replication (lenacapavir [153, 154]), and nonnucleoside reverse transcriptase inhibitors (NNRTIs) that induce cytotoxicity in infected cells (ie, targeted activator kill, TACK [155]), which have expanded the armamentarium of effective antiretroviral strategies. While inconsistent ART adherence leading to resistance remains problematic, advances in the development of investigational long-acting, slow-effective release (LASER) ART may enable many ART-suppressed PLWH to transition from daily oral regimens to FDA-approved injectable regimens administered monthly (cabotegravir plus rilpivirine) [156–158] or regimens including twice yearly injections (lenacapavir) [154, 159, 160].
If ART suppression of virus replication permits a stable disease course without comorbidities and blocks viral transmission, can this be considered as remission? Or if a cure is required to reduce stigmatization, how does one balance this need against the potential for therapy-related reductions in quality of life, particularly if long-term maintenance therapy is required? Likewise, assuming a prospective cure does not prevent subsequent re-infection, should all persons who experience a cure be maintained on antiretroviral agents? The advances in treatment of HIV as a chronic disease should substantially raise the bar by which the effectiveness and suitability of cure strategies are judged.
Although we have not yet identified a scalable strategy to control or cure HIV, significant progress has been made in that direction. Thus far, only CCR5D32/D32 alloHSCT has resulted in a reproducible cure [113–119], but this strategy is limited by the availability of CCR5D32/D32 HLA-matched donors and significant morbidity and mortality of the allogeneic transplant recipient [120–123]. The transplantation of autologous gene-edited CD4+ T cells has, to date, demonstrated limited efficacy [135, 136], but might be more effective if a selection process (perhaps even periods of ART cessation) promotes their expansion. Other promising approaches will likely use bNAbs in combination with immune modulators to enhance antiviral immunity to selectively intercept and eliminate cells supporting viral replication upon ART cessation. Other compelling combination approaches may be feasible but would require further study and may be complicated by safety concerns: ie, CCR5WT/WT alloHSCT with a CCR5 inhibitor (eg, Maraviroc or Leronlimab) [141] or CCR5D32/D32 CD4+ chimeric antigen receptor (CAR) T cells directed against multiple bNAb targets [161]. While there is optimism regarding the potential of combination approaches, it is important to acknowledge that a scalable, clinically well-tolerated therapeutic strategy to either cure or control HIV in the absence of ART is unlikely to occur in the same timescale as has been proposed to end the HIV epidemic (ie, by 2030) [6, 8]. As such, an emerging area of concern is to address how age-related perturbations in immune function and HIV-associated immunosenescence [162–164] intersect with the maintenance of infected cells during long-term ART [165], with the risk of co-morbidities [166–168], and with responsiveness to immune-based cure strategies [169–171]. Moreover, a portfolio of experimental and FDA-approved drugs are under investigation for their ability to either eliminate (ie, senolytics) or alter the function (ie, senomorphic) of senescent cells, including Bcl-2 inhibitors (Venetoclax), JAK1/JAK2 inhibitors (ruxolitinib), mTOR inhibitors (rapamycin), and tyrosine kinase inhibitors (dasatinib) among others [172, 173]; however, further study is required to evaluate these agents for their utility in attenuating or reversing HIV-induced immunosenescence and affecting HIV persistence.
As advances in ART have rendered HIV a manageable chronic disease, we may need to reconsider what is acceptable in pursuit of a cure. ART-suppressed PLWH remain at a small, but demonstrably greater, risk of comorbidities [174], but it is unclear, and a key question the field will need to address, if a cure will diminish that risk or if immune responses are “scarred” due to persistent epigenetic remodeling [175, 176] or other irreversible damage. Additionally, as cure strategies are considered and developed, effects on quality of life and life expectancy must be monitored. Indeed, while morbidities of FDA-approved immunotherapies [177, 178] and myeloablative conditioning [179] are acceptable in life-threatening malignancies, that will not be the case for most PLWH. Moreover, immunotherapy-based approaches may entail long-term maintenance therapy resulting in substantial “financial toxicity” [180–182] relative to modern ART regimens. Likewise, given their high projected out-of-pocket cost [183] and requirements for significant clinical infrastructure for implementation and adverse event management, it is doubtful that many cure strategies could be successfully implemented in resource-poor settings and may not be a realistic option for all, at least in the immediate future. To circumvent these limitations, gene editing approaches using CRISPR-Cas9 and genome-integrating viral vectors are being explored to induce long-term endogenous gene expression, a strategy that has been successfully employed to treat sickle cell disease (eg, FDA-approved Casgevy and Lyfgenia) [184, 185]. For example, gene transfer using adeno-associated virus (AAV) vectors has been explored to induce long-term production of bNAbs; yet, while initial results in the NHP model were encouraging (eg, the Miami monkey [186]), efficacy in humans is limited by the generation of anti-drug antibodies (ADAs) and baseline resistance [187, 188]. Likewise, sustained viral control is rare following the passive transfer of bNAbs in PLWH [106, 107]. A less durable alternative, the delivery of mRNA [189, 190] via lipid nanoparticles (LNPs), has been demonstrated as a safe, effective, and economical therapeutic option to temporarily induce transgene expression, as evidenced by the success of the SARS-CoV-2 mRNA vaccines [191, 192]. When combined with emergent modification strategies to target LNPs to specific organs [193, 194], this may represent a compelling approach through which to transiently modulate innate, adaptive, or humoral immune responses.
It is important to recognize that the timeline for developing an effective, simple, and inexpensive cure strategy is unknown and that this strategy may initially result in a net benefit for some, but not all PLWH. Despite these formidable challenges in finding a scalable curative approach for most PLWH, there remains broad interest and need in the community and among HIV researchers for exploring novel therapeutic strategies to cure HIV. Furthermore, as proven for the past 40 years, research in HIV will continue to benefit many fields in innovating science, including the development of cutting-edge diagnostic technologies and treatment modalities for chronic diseases. In the meantime, as many novel strategies will target host elements, PLWH considering engaging in these trials will need to have a realistic understanding of the risks of these approaches as they pursue a cure. We provide below the perspectives of several leading researchers in the field regarding the status and future of HIV cure research.
The past several years of HIV cure research have seen remarkable novel immunotherapeutic strategies and unfortunate clinical trial setbacks that together complicate the potential for the development of a truly scalable HIV-eradicative cure. Our aspirational goal continues to be a “one pill” therapeutic HIV cure strategy like that available for hepatitis C virus (HCV). However, there does not yet appear to be a simple virological approach to eliminating the entirety of the latent HIV reservoir, at least until gene editing technologies can be developed capable of finding and eliminating the integrated virus from every infected cell in the body. As such, we must consider the value of a stopgap immunological-based functional cure versus the end goal of pharmacological eradicative cure. Like other chronic human viral infections, most notably the herpesvirus family, HIV co-opts host-preserving immunological mechanisms inherently present at the cellular and anatomical level to perpetuate the viral reservoir and prevent its immunological clearance. Simply reinvigorating an already failed immune response through T cell restimulation, NK cell modulation, bNAb administration, or exogenous cell therapy is unlikely to overcome these issues. The immune system cannot identify and eliminate HIV-infected cells if (1) the virus remains latent, (2) the infected cells are resistant to cell death, and (3) immune effectors with appropriate functional properties cannot access infected cells. Emerging technologies, including CRISPR-based gene editing and therapeutic RNA-based immunomodulation, hold promise to address these problems, but until we develop immunomodulatory strategies that effectively circumvent biology, even a functional cure for HIV will remain elusive.
What can the human immune system do against HIV reservoir cells – the cells that persist despite ART and represent the main barrier to a cure? The traditional answer to this question has been “nothing,” because, in theory, these cells are in a resting state that promotes proviral transcriptional silencing and maintains “viral latency,” protecting these cells from immune recognition. I believe that this concept, which has now been pursued for more than 2 decades, is in need of a profound revision: Newer single-cell or single-genome assays suggest that these cells can be surprisingly vulnerable to immune defenses and that signatures and footprints of immune selection in the viral reservoir cell pool are remarkably evident when high-resolution analysis techniques are used. I think that we now have the proper tools to investigate the immune mechanisms that can recognize and target HIV reservoir cells and that doing so will ultimately permit us to find weaknesses and vulnerabilities of viral reservoir cells that can be effectively exploited for clinical cure interventions. Key to the success of these efforts will be listening to and learning from community members, so that HIV cure research is a project “of the people, by the people, and for the people” living with HIV.
The last decade has shown that the mechanisms leading to remission of HIV differ in some aspect from those of natural control of HIV. Research in recent years highlighted a potentially important role for innate immunity in post-treatment control. While the IFN-a response is not sufficient for avoiding viral rebound, the pressure it exerts on the virus might give the host more time to chime in with additional antiviral arms. Cellular components of the innate immune system, in particular NK cells, might act directly on eliminating reservoir cells. This can probably only happen if viral transcription is active, such as during residual viral replication, replication in anatomical reservoirs, and at treatment interruption, when the virus is in the process of rebounding. Interestingly, NK cells have the potential to migrate into anatomical sanctuaries, such as B cell follicles. NK cells also provide the necessary help to neutralizing antibodies for ADCC. Moreover, their capacity to adapt and develop antigen-specific memory might revolutionize immunotherapies harnessing NK cells. Whether this property can be exploited for HIV cure approaches, for instance through vaccinal or immunotherapies, still needs to be further tested. Even if most of the PLWH do not develop highly efficient NK cells for several reasons, off-the-shelf products could be constructed that could substitute. Transfer of allogeneic NK and chimeric antigen receptor (CAR)-NK cells present a lower risk of GVHD or cytokine release syndrome than CAR T cells. CAR-NK cells are not susceptible to infection and are more easily applicable for off-the-shelf usage as they do not require a strict autologous HLA matching. Collectively, the arsenal of tools that is available for developing an effective cure can be enlarged by harnessing innate immunity, increasing the chances of achieving a scalable cure one day.
In the last 8 years, much has changed, but some challenges remain daunting. The effort to end the epidemic using tools at hand to treat all PLWH and completely interrupt ongoing transmission is critical but will not address the desires of millions of PLWH. However, significant advances in the understanding of persistent infection, technologies to measure and characterize the latent reservoir, and approaches to attack the sources of viral rebound after ATI have been made. A substantial number of demanding clinical studies have been successfully completed, adding to our knowledge without measurable harm to the altruistic participants who have made this work possible.
We must safely and methodically seek to enlarge on these modest initial advances, while also developing new approaches. A broadly scalable cure strategy is obviously the ultimate goal, but in the near-term, we hope to see proof-of-concept studies that demonstrate the ability to substantially deplete persistent infection using serial latency reversal agents combined with engineered immunotherapies. Ongoing efforts to develop new approaches, such as base editing of proviral DNA, and interventions to blunt the entry of provirus into latency during ART initiation, are likely to contribute to a later wave of clinical advances towards ART-free remission. If we are as persistent as the virus, we are certain to make progress.
Since publication of the previous salon, the field has made important discoveries in HIV reservoir dynamics that inform HIV cure strategies currently in clinical trials. First, data indicating that the majority of the persistent CD4+ T cell reservoir is established at or near ART initiation have fueled current interventions. Next, HIV-specific immune responses appear to drive decreases in the intact proviral reservoir over the first 5 to 10 years of ART suppression. While insufficient, the consistent trend towards early reservoir decrease implies a baseline level of immune clearance that could be enhanced. Finally, there is evidence that following the early reservoir decline, immune exhaustion, reservoir cells resistant to killing, and cellular proliferation outcompete anti-HIV immune responses leading to stagnation of reservoir clearance. While challenging, this body of work identifies plausible targets of current interventions. With advances in related fields of vaccinology, cancer immunology, and genetic engineering, current trials aim to block reservoir formation at ART initiation, enhance HIV-specific cellular and humoral immunity via vaccination, immunomodulation, or gene therapy, and limit cellular proliferation. Thus, the goal of a safe, effective, and scalable intervention to induce HIV cure or ART-free virus control remains a substantial, long-term challenge, but I believe we can celebrate the field’s tangible strides towards understanding the determinants of HIV persistence as they better illuminate the path forward.
At the start of 1928, the world had no idea that Dr. Alexander Fleming was about to discover penicillin and open the antibiotic era. At the beginning of 2007, the world had no idea that Timothy Ray Brown was about to undergo his stem cell transplant and that an HIV cure was possible. The arc of scientific discovery is fundamentally not a linear process, and the timeline for discovering an HIV cure and ART-free HIV control is unpredictable. Having said that, I am optimistic about the future. On the basic science side, new single-cell techniques are giving us unprecedented resolution of the cellular transcriptomic, proteomic, and metabolic pathways that underpin HIV persistence. This fundamental knowledge is critical to the development of new strategies. In addition, there are a number of exciting approaches being tested in clinical trials, including bNAbs, latency-reversing agents, immunomodulators, HIV silencing strategies, and gene modification. I can’t wait to see what 2024 (and beyond) will bring for the field and for our patients!
While ART has made HIV disease manageable for many, this is not the case for most – especially for those in resource-limited parts of the world where the prevalence of disease is high, adherence is difficult, and the availability of ART is increasingly uncertain. For these individuals, it is important to find a way to maintain durable viral suppression absent guaranteed provision of and/or long-term adherence to ART [195].
This goal appears to be technically within reach. “Single shot” (in vivo) interventions targeting the liver are already poised to provide clinical benefit for millions living with chronic conditions such as hemophilia [196] and hyperlipidemia [197]. Emerging in parallel are vectors enabling in vivo targeting of long-lived cells that are even more relevant to an HIV “cure,” eg, hematopoietic stem cells, B cells, and TSCM cells. Innovative approaches to edit or add genes using only mRNA are opening the door to insertion of membrane-associated and secreted Env-antagonists into the safe harbor of CCR5, thereby also knocking it out. Better understanding of protective CD8+ T cell responses in “elite controllers” who suppress HIV absent ART [198] suggests the design of therapeutic vaccines to induce analogous, suppressive T cell responses in non-controllers. Most likely in combination, low-touch interventions such as these may well provide durable viral suppression in the absence of ART.
If so, it will then be necessary to assure accessibility, affordability, and acceptability. While the COVID pandemic has demonstrated that it is possible to scale non-viral vectors carrying mRNA cargos, it has also highlighted gross iniquities in healthcare distribution around the world. Eradication of HIV disease will require the distribution of “curative interventions” that prevent disease progression, block infection upon re-exposure [199], and curtail transmission to those at risk. Aspirational as this goal may seem to be, now is the time for partnerships to form [200, 201] so that such interventions can be advanced for the benefit of all.
Over the last 8 years, progress in cure research has been substantial. These advances have been significantly accelerated by new technologies, such as single-cell sequencing, and in the future, I anticipate further advances as a result of mRNA and lipid nanoparticle technologies that can be used to deliver gene editing directly in vivo. In work led by the International AIDS Society to define a target product profile for an HIV cure, which included widespread consultation with the community, the bar is high. There is an expectation that a cure intervention will have minimal toxicity, high efficacy, and also protect from re-infection. I feel we are far off achieving this currently. However, there is no doubt we have made some advances. Several clinical trials of immunomodulatory interventions, administered at the time of viremia (either at ART initiation or ART interruption), have been shown to induce viral control in roughly one third of participants. This has been described with combination bNAbs either alone or with another immunomodulatory agent and more recently in a small randomized clinical trial of low-dose anti-PD-1 mAbs. To me, these findings are exciting, as it is the first positive signal showing it is possible to induce viral control in some PLWH. There is much more work to be done to understand the mechanism of control and why only some PLWH can achieve this outcome. I am also excited about the advances in in vivo gene therapy using viral vectors, but in the future, we will be using mRNA. The induction of long-term antibody production using insertional gene therapy is feasible and showing promise in some macaque models. No matter how hard or complex the science, a cure is of no value if it cannot be scaled and available globally at a reasonable cost. These features must remain front and center, at the same time as we advance the science.
The persistent inflammation and immune activation associated with HIV infection not only can lead to T cell exhaustion and senescence but also can complicate the development of latency reversal treatments that rely on T cell responses for both the induction of viral replication from its latent state and the elimination of these reactivated latently infected cells by either viral cytopathic effect or immune cell-mediated killing. However, as our understanding of HIV persistence has increased, so too has my optimism for an HIV cure. By specifically characterizing alterations in cellular signaling and metabolic functions that lead to the chronic inflammatory environment and immune cell activation, we can identify targets for therapeutic interventions that may ameliorate the environment for HIV to persist. For example, many of the processes we observe in HIV infection, such as aberrant DNA damage and repair, shortened telomeres, and impaired mitochondrial function, are also associated with biological aging. Recognizing the parallels between HIV and other biological and disease processes provides opportunity to adopt therapeutic interventions that have shown efficacy for other pathologies. As the pace of discovery and development of new technology accelerates, so will our progress to a cure.
Recognizing that complete eradication of HIV is a daunting task and may not now be feasible with the currently available knowledge and technical tools, I am, however, optimistic that we can achieve a “functional cure” during our lifetimes. That is, “a state in which the virus can still be detected, but ART may be withdrawn without virus recrudescence.” Although multiple thoughtful approaches are being pursued, I am convinced that only a combination of strategies will be effective and generalizable to achieve a functional cure. A more comprehensive understanding of the immune system, greatly fueled by ongoing research, and new tools for genetic editing and cell engineering have resulted in significant advances and informative attempts to achieve this objective by using a combination of strategies to manipulate immune cells to be both HIV resistant and more HIV responsive, to allow effector cells to reach anatomical sites rich in HIV reservoirs, to revert (or reinforce!) epigenetic mechanisms of HIV latency, and to counteract virus diversity and resistance / escape.
A challenge remains on how scalable a functional cure strategy will be. Considering the successful examples of the Berlin and London patients, in whom HIV remission was achieved through transplantation of allogeneic cells resistant to HIV infection, it may be possible that curing HIV may require highly personalized, technically demanding, and costly strategies, for which scale is a barrier, considering that 39 million people are living with HIV. The countries most affected by the HIV epidemic may not have resources and infrastructure to mass produce genetically modified cells and other biologics required in these strategies. Beyond the huge basic and translational science issues presented by HIV cure research, implementation science and health equity issues need also to be considered.
Finally, a close interaction with different global communities to recognize their views and visions of HIV cure is necessary. Managing expectations is also important, considering the stigma that living with HIV and taking ART still represent in many parts of the world. Importantly, maintaining and even increasing the efforts and investments on HIV cure research is of capital importance. The relevance and possible risk of participating in HIV cure research needs to be clearly stated and communicated, not only in clinical trials assessing the effectiveness of specific cure strategies, but also in basic research, aiming to gain additional knowledge on HIV persistence and immunopathogenesis, which often involves accessing tissue samples by use of invasive procedures.
Community engagement in basic science is essential to the success of finding an HIV cure. Community engagement in HIV research has evolved and become a core component of ethical, meaningful, and impactful involvement of individuals and organizations that share a common stake in the effort to find solutions to minimize and eventually eradicate HIV. More importantly, for PLWH, the stakes are high for finding a permanent solution that relieves them of the lifelong burden of a costly medical routine, social stigma, discrimination, and other unique challenges associated with HIV. It takes all players working in creative and collaborative ways to find the cure that will finally and permanently end HIV as a global threat to the wellbeing and quality of life for people who are wedged in the margins of privilege, power, and safety. It has been demonstrated that research in HIV has led the way across many fields of research in innovating science, technology, care, treatment, prevention, and advocacy for health equity and rights. To be sure, if we are to sustain a successful biomedical solution, one way or another, all roads to the end of HIV go through communities, especially those most affected by the epidemic.
Effective community engagement can involve: (1) coordination of eradication strategies in the community; (2) capacity-building with community advisors, strategic partners, and researchers to learn from each other and work together as collaborators; (3) communication of information, education, and learning with the researchers, constituents, and stakeholders; and (4) creation of new pathways for shared learning, translational experiences, and partnership activities. Collaborative approaches, and commitment to serving as a bridge from science to society, from molecules to medicine, can be the heart of community organizations’ long-term, intentional relationships with a diversity of laboratory, clinical, and community-based researchers who, together, continue to do everything possible to find a workable cure to HIV and an end to the epidemic.
Images were created using BioRender.com.
JH and MP are supported by funding from the Enterprise for Research and Advocacy to Stop and Eradicate (ERASE) HIV Martin Delaney Collaboratory co-funded by National Heart, Lung, and Blood Institute; National Institute of Diabetes, Digestive, and Kidney Diseases; National Institute of Neurological Disorders and Stroke; National Institute on Drug Abuse; and the National Institute of Allergy and Infectious Diseases (UM1AI164562).
JH and MP have active collaborations with Merck & Co., Inc., and routinely receive antiretroviral compounds for nonhuman primate studies from ViiV Healthcare and Gilead Sciences, but the authors declare no financial stake. MML has received competitive grant funding from Gilead.
1. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Cottle L, Zhang XC, Makhema J, Mills LA, Panchia R, Faesen S, Eron J, Gallant J, Havlir D, Swindells S, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano DD, Essex M, Hudelson SE, Redd AD, Fleming TR, Team HS. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830-9. Epub 20160718. doi: 10.1056/NEJMoa1600693. PubMed PMID: 27424812; PMCID: PMC5049503.
2. Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, Degen O, Corbelli GM, Estrada V, Geretti AM, Beloukas A, Raben D, Coll P, Antinori A, Nwokolo N, Rieger A, Prins JM, Blaxhult A, Weber R, Van Eeden A, Brockmeyer NH, Clarke A, Del Romero Guerrero J, Raffi F, Bogner JR, Wandeler G, Gerstoft J, Gutierrez F, Brinkman K, Kitchen M, Ostergaard L, Leon A, Ristola M, Jessen H, Stellbrink HJ, Phillips AN, Lundgren J, Group PS. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428-38. Epub 20190502. doi: 10.1016/S0140-6736(19)30418-0. PubMed PMID: 31056293; PMCID: PMC6584382.
3. UNAIDS. Global HIV & AIDS statistics — Fact sheet 2022 [cited 2023 October 2]. Available from: https://www.unaids.org/en/resources/fact-sheet.
4. Edwards JK, Cole SR, Breger TL, Rudolph JE, Filiatreau LM, Buchacz K, Humes E, Rebeiro PF, D’Souza G, Gill MJ, Silverberg MJ, Mathews WC, Horberg MA, Thorne J, Hall HI, Justice A, Marconi VC, Lima VD, Bosch RJ, Sterling TR, Althoff KN, Moore RD, Saag M, Eron JJ. Mortality Among Persons Entering HIV Care Compared With the General U.S. Population : An Observational Study. Ann Intern Med. 2021;174(9):1197-206. Epub 20210706. doi: 10.7326/M21-0065. PubMed PMID: 34224262; PMCID: PMC8453103.
5. Center for Disease Control and Prevention. HIV Declines Among Young People and Drives Overall Decrease in New HIV Infections [cited 2023 October 2]. Available from: https://www.cdc.gov/media/releases/2023/p0523-hiv-declines-among-young-people.html.
6. UNAIDS. The Path that Ends AIDS [cited 2023 October 2]. Available from: https://www.unaids.org/sites/default/files/media_asset/2023-unaids-global-aids-update_en.pdf.
7. Stover J, Glaubius R, Teng Y, Kelly S, Brown T, Hallett TB, Revill P, Barnighausen T, Phillips AN, Fontaine C, Frescura L, Izazola-Licea JA, Semini I, Godfrey-Faussett P, De Lay PR, Benzaken AS, Ghys PD. Modeling the epidemiological impact of the UNAIDS 2025 targets to end AIDS as a public health threat by 2030. PLoS Med. 2021;18(10):e1003831. Epub 20211018. doi: 10.1371/journal.pmed.1003831. PubMed PMID: 34662333; PMCID: PMC8559943.
8. Office of Infectious Disease and HIV/AIDS Policy. Ending the HIV Epidemic [cited 2024 February 6]. Available from: https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview/.
9. Gray R, McManus H, King J, Petoumenos K, Grulich A, Guy R, McGregor S, editors. Australia’s progress towards ending HIV as a public health threat: trends in epidemiological metrics over 2004-2021. IAS; 2023 July 24; Brisbane, Australia.
10. Lederman MM, Cannon PM, Currier JS, June CH, Kiem HP, Kuritzkes DR, Lewin SR, Margolis DM, McCune JM, Mellors JW, Schacker TW, Sekaly RP, Tebas P, Walker BD, Douek DC. A Cure for HIV Infection: “Not in My Lifetime” or “Just Around the Corner”? Pathog Immun. 2016;1(1):154-64. doi: 10.20411/pai.v1i1.133. PubMed PMID: 27668293; PMCID: PMC5033048.
11. Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727-8. Epub 20030518. doi: 10.1038/nm880. PubMed PMID: 12754504.
12. Li JZ, Aga E, Bosch RJ, Pilkinton M, Kroon E, MacLaren L, Keefer M, Fox L, Barr L, Acosta E, Ananworanich J, Coombs R, Mellors JW, Landay AL, Macatangay B, Deeks S, Gandhi RT, Smith DM. Time to Viral Rebound After Interruption of Modern Antiretroviral Therapies. Clin Infect Dis. 2022;74(5):865-70. doi: 10.1093/cid/ciab541. PubMed PMID: 34117753; PMCID: PMC8906742.
13. Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, Kuritzkes DR, Lederman MM, Para M, Gandhi RT. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016;30(3):343-53. doi: 10.1097/QAD.0000000000000953. PubMed PMID: 26588174; PMCID: PMC4840470.
14. Shan L, Deng K, Gao H, Xing S, Capoferri AA, Durand CM, Rabi SA, Laird GM, Kim M, Hosmane NN, Yang HC, Zhang H, Margolick JB, Li L, Cai W, Ke R, Flavell RA, Siliciano JD, Siliciano RF. Transcriptional Reprogramming during Effector-to-Memory Transition Renders CD4(+) T Cells Permissive for Latent HIV-1 Infection. Immunity. 2017;47(4):766-75 e3. doi: 10.1016/j.immuni.2017.09.014. PubMed PMID: 29045905; PMCID: PMC5948104.
15. Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512(7512):74-7. Epub 20140720. doi: 10.1038/nature13594. PubMed PMID: 25042999; PMCID: PMC4126858.
16. Whitney JB, Lim SY, Osuna CE, Kublin JL, Chen E, Yoon G, Liu PT, Abbink P, Borducci EN, Hill A, Lewis MG, Geleziunas R, Robb ML, Michael NL, Barouch DH. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat Commun. 2018;9(1):5429. Epub 20181221. doi: 10.1038/s41467-018-07881-9. PubMed PMID: 30575753; PMCID: PMC6303321.
17. Gantner P, Buranapraditkun S, Pagliuzza A, Dufour C, Pardons M, Mitchell JL, Kroon E, Sacdalan C, Tulmethakaan N, Pinyakorn S, Robb ML, Phanuphak N, Ananworanich J, Hsu D, Vasan S, Trautmann L, Fromentin R, Chomont N. HIV rapidly targets a diverse pool of CD4(+) T cells to establish productive and latent infections. Immunity. 2023;56(3):653-68 e5. Epub 20230217. doi: 10.1016/j.immuni.2023.01.030. PubMed PMID: 36804957; PMCID: PMC10023508.
18. Abrahams MR, Joseph SB, Garrett N, Tyers L, Moeser M, Archin N, Council OD, Matten D, Zhou S, Doolabh D, Anthony C, Goonetilleke N, Karim SA, Margolis DM, Pond SK, Williamson C, Swanstrom R. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci Transl Med. 2019;11(513). doi: 10.1126/scitranslmed.aaw5589. PubMed PMID: 31597754; PMCID: PMC7233356.
19. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR, Team HS. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493-505. Epub 20110718. doi: 10.1056/NEJMoa1105243. PubMed PMID: 21767103; PMCID: PMC3200068.
20. Takata H, Buranapraditkun S, Kessing C, Fletcher JL, Muir R, Tardif V, Cartwright P, Vandergeeten C, Bakeman W, Nichols CN, Pinyakorn S, Hansasuta P, Kroon E, Chalermchai T, O’Connell R, Kim J, Phanuphak N, Robb ML, Michael NL, Chomont N, Haddad EK, Ananworanich J, Trautmann L, Rv254/Search, the RVSSG. Delayed differentiation of potent effector CD8(+) T cells reducing viremia and reservoir seeding in acute HIV infection. Sci Transl Med. 2017;9(377). doi: 10.1126/scitranslmed.aag1809. PubMed PMID: 28202771; PMCID: PMC5678930.
21. Shelton EM, Reeves DB, Bender Ignacio RA. Initiation of Antiretroviral Therapy during Primary HIV Infection: Effects on the Latent HIV Reservoir, Including on Analytic Treatment Interruptions. AIDS Rev. 2020;23(1):28-39. doi: 10.24875/AIDSRev.20000001. PubMed PMID: 33105471; PMCID: PMC7987773.
22. Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, O’Connell RJ, Rupert A, Chomont N, Valcour V, Kim JH, Robb ML, Michael NL, Douek DC, Ananworanich J, Utay NS, Rv254/Search RS, teams Sp. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin Infect Dis. 2017;64(2):124-31. Epub 20161012. doi: 10.1093/cid/ciw683. PubMed PMID: 27737952; PMCID: PMC5215214.
23. Muccini C, Crowell TA, Kroon E, Sacdalan C, Ramautarsing R, Seekaew P, Phanuphak P, Ananworanich J, Colby DJ, Phanuphak N. Leveraging early HIV diagnosis and treatment in Thailand to conduct HIV cure research. AIDS Res Ther. 2019;16(1):25. Epub 20190906. doi: 10.1186/s12981-019-0240-4. PubMed PMID: 31492161; PMCID: PMC6729012.
24. Okoye AA, Hansen SG, Vaidya M, Fukazawa Y, Park H, Duell DM, Lum R, Hughes CM, Ventura AB, Ainslie E, Ford JC, Morrow D, Gilbride RM, Legasse AW, Hesselgesser J, Geleziunas R, Li Y, Oswald K, Shoemaker R, Fast R, Bosche WJ, Borate BR, Edlefsen PT, Axthelm MK, Picker LJ, Lifson JD. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat Med. 2018;24(9):1430-40. Epub 20180806. doi: 10.1038/s41591-018-0130-7. PubMed PMID: 30082858; PMCID: PMC6389357.
25. Colby DJ, Trautmann L, Pinyakorn S, Leyre L, Pagliuzza A, Kroon E, Rolland M, Takata H, Buranapraditkun S, Intasan J, Chomchey N, Muir R, Haddad EK, Tovanabutra S, Ubolyam S, Bolton DL, Fullmer BA, Gorelick RJ, Fox L, Crowell TA, Trichavaroj R, O’Connell R, Chomont N, Kim JH, Michael NL, Robb ML, Phanuphak N, Ananworanich J, group RVs. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923-6. Epub 20180611. doi: 10.1038/s41591-018-0026-6. PubMed PMID: 29892063; PMCID: PMC6092240.
26. Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, Kearney MF, Anderson EM, Buchbinder SP, Cohen SE, Abdel-Mohsen M, Pohlmeyer CW, Fromentin R, Hoh R, Liu AY, McCune JM, Spindler J, Metcalf-Pate K, Hobbs KS, Thanh C, Gibson EA, Kuritzkes DR, Siliciano RF, Price RW, Richman DD, Chomont N, Siliciano JD, Mellors JW, Yukl SA, Blankson JN, Liegler T, Deeks SG. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS Med. 2017;14(11):e1002417. Epub 20171107. doi: 10.1371/journal.pmed.1002417. PubMed PMID: 29112956; PMCID: PMC5675377.
27. Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871-9. doi: 10.1097/00002030-200309050-00005. PubMed PMID: 12960819.
28. McMyn NF, Varriale J, Fray EJ, Zitzmann C, MacLeod H, Lai J, Singhal A, Moskovljevic M, Garcia MA, Lopez BM, Hariharan V, Rhodehouse K, Lynn K, Tebas P, Mounzer K, Montaner LJ, Benko E, Kovacs C, Hoh R, Simonetti FR, Laird GM, Deeks SG, Ribeiro RM, Perelson AS, Siliciano RF, Siliciano JM. The latent reservoir of inducible, infectious HIV-1 does not decrease despite decades of antiretroviral therapy. J Clin Invest. 2023;133(17). Epub 20230901. doi: 10.1172/JCI171554. PubMed PMID: 37463049; PMCID: PMC10471168.
29. Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893-900. Epub 20090621. doi: 10.1038/nm.1972. PubMed PMID: 19543283; PMCID: PMC2859814.
30. Mendoza P, Jackson JR, Oliveira TY, Gaebler C, Ramos V, Caskey M, Jankovic M, Nussenzweig MC, Cohn LB. Antigen-responsive CD4+ T cell clones contribute to the HIV-1 latent reservoir. J Exp Med. 2020;217(7). doi: 10.1084/jem.20200051. PubMed PMID: 32311008; PMCID: PMC7336300.
31. Simonetti FR, Zhang H, Soroosh GP, Duan J, Rhodehouse K, Hill AL, Beg SA, McCormick K, Raymond HE, Nobles CL, Everett JK, Kwon KJ, White JA, Lai J, Margolick JB, Hoh R, Deeks SG, Bushman FD, Siliciano JD, Siliciano RF. Antigen-driven clonal selection shapes the persistence of HIV-1-infected CD4+ T cells in vivo. J Clin Invest. 2021;131(3). doi: 10.1172/JCI145254. PubMed PMID: 33301425; PMCID: PMC7843227.
32. Collora JA, Liu R, Pinto-Santini D, Ravindra N, Ganoza C, Lama JR, Alfaro R, Chiarella J, Spudich S, Mounzer K, Tebas P, Montaner LJ, van Dijk D, Duerr A, Ho YC. Single-cell multiomics reveals persistence of HIV-1 in expanded cytotoxic T cell clones. Immunity. 2022;55(6):1013-31 e7. Epub 20220322. doi: 10.1016/j.immuni.2022.03.004. PubMed PMID: 35320704; PMCID: PMC9203927.
33. Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science. 2014;345(6193):179-83. Epub 20140626. doi: 10.1126/science.1254194. PubMed PMID: 24968937; PMCID: PMC4262401.
34. Wagner TA, McLaughlin S, Garg K, Cheung CY, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, Frenkel LM. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science. 2014;345(6196):570-3. Epub 20140710. doi: 10.1126/science.1256304. PubMed PMID: 25011556; PMCID: PMC4230336.
35. Coffin JM, Bale MJ, Wells D, Guo S, Luke B, Zerbato JM, Sobolewski MD, Sia T, Shao W, Wu X, Maldarelli F, Kearney MF, Mellors JW, Hughes SH. Integration in oncogenes plays only a minor role in determining the in vivo distribution of HIV integration sites before or during suppressive antiretroviral therapy. PLoS Pathog. 2021;17(4):e1009141. Epub 20210407. doi: 10.1371/journal.ppat.1009141. PubMed PMID: 33826675; PMCID: PMC8055010.
36. Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, Pereyra F, Zurakowski R, Walker BD, Rosenberg ES, Yu XG, Lichterfeld M. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014;20(2):139-42. Epub 20140112. doi: 10.1038/nm.3445. PubMed PMID: 24412925; PMCID: PMC3959167.
37. Jaafoura S, de Goer de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, Krzysiek R, Merad M, Seng R, Tardieu M, Delfraissy JF, Goujard C, Taoufik Y. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4(+) memory T Cells. Nat Commun. 2014;5:5407. Epub 20141110. doi: 10.1038/ncomms6407. PubMed PMID: 25382623; PMCID: PMC4241984.
38. Einkauf KB, Lee GQ, Gao C, Sharaf R, Sun X, Hua S, Chen SM, Jiang C, Lian X, Chowdhury FZ, Rosenberg ES, Chun TW, Li JZ, Yu XG, Lichterfeld M. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest. 2019;129(3):988-98. Epub 20190128. doi: 10.1172/JCI124291. PubMed PMID: 30688658; PMCID: PMC6391088.
39. Lian X, Seiger KW, Parsons EM, Gao C, Sun W, Gladkov GT, Roseto IC, Einkauf KB, Osborn MR, Chevalier JM, Jiang C, Blackmer J, Carrington M, Rosenberg ES, Lederman MM, McMahon DK, Bosch RJ, Jacobson JM, Gandhi RT, Peluso MJ, Chun TW, Deeks SG, Yu XG, Lichterfeld M. Progressive transformation of the HIV-1 reservoir cell profile over two decades of antiviral therapy. Cell Host Microbe. 2023;31(1):83-96 e5. Epub 20230102. doi: 10.1016/j.chom.2022.12.002. PubMed PMID: 36596305; PMCID: PMC9839361.
40. Einkauf KB, Osborn MR, Gao C, Sun W, Sun X, Lian X, Parsons EM, Gladkov GT, Seiger KW, Blackmer JE, Jiang C, Yukl SA, Rosenberg ES, Yu XG, Lichterfeld M. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell. 2022;185(2):266-82 e15. Epub 20220112. doi: 10.1016/j.cell.2021.12.011. PubMed PMID: 35026153; PMCID: PMC8809251.
41. Deleage C, Wietgrefe SW, Del Prete G, Morcock DR, Hao XP, Piatak M, Jr., Bess J, Anderson JL, Perkey KE, Reilly C, McCune JM, Haase AT, Lifson JD, Schacker TW, Estes JD. Defining HIV and SIV Reservoirs in Lymphoid Tissues. Pathog Immun. 2016;1(1):68-106. doi: 10.20411/pai.v1i1.100. PubMed PMID: 27430032; PMCID: PMC4943335.
42. Liu Z, Julius P, Kang G, West JT, Wood C. Subtype C HIV-1 reservoirs throughout the body in ART-suppressed individuals. JCI Insight. 2022;7(20). Epub 20221024. doi: 10.1172/jci.insight.162604. PubMed PMID: 36278485; PMCID: PMC9714794.
43. Dube M, Tastet O, Dufour C, Sannier G, Brassard N, Delgado GG, Pagliuzza A, Richard C, Nayrac M, Routy JP, Prat A, Estes JD, Fromentin R, Chomont N, Kaufmann DE. Spontaneous HIV expression during suppressive ART is associated with the magnitude and function of HIV-specific CD4(+) and CD8(+) T cells. Cell Host Microbe. 2023;31(9):1507-22 e5. doi: 10.1016/j.chom.2023.08.006. PubMed PMID: 37708853; PMCID: PMC10542967.
44. Takata H, Mitchell JL, Pacheco J, Pagliuzza A, Pinyakorn S, Buranapraditkun S, Sacdalan C, Leyre L, Nathanson S, Kakazu JC, Intasan J, Prueksakaew P, Chomchey N, Phanuphak N, de Souza M, Haddad EK, Rolland M, Tovanabutra S, Vasan S, Hsu DC, Chomont N, Trautmann L, Rv254/Search, Rv304/Search. An active HIV reservoir during ART is associated with maintenance of HIV-specific CD8(+) T cell magnitude and short-lived differentiation status. Cell Host Microbe. 2023;31(9):1494-506 e4. doi: 10.1016/j.chom.2023.08.012. PubMed PMID: 37708852; PMCID: PMC10564289.
45. Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, Giavedoni LD, Lebon P, Barre-Sinoussi F, Benecke A, Muller-Trutwin MC. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119(12):3544-55. doi: 10.1172/JCI40093. PubMed PMID: 19959873; PMCID: PMC2786805.
46. Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G. Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nat Med. 2011;17(7):830-6. Epub 20110626. doi: 10.1038/nm.2395. PubMed PMID: 21706028; PMCID: PMC3253129.
47. Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, Reeves RK, Derreudre-Bosquet N, Muller-Trutwin M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med. 2017;23(11):1277-86. Epub 20171016. doi: 10.1038/nm.4421. PubMed PMID: 29035370; PMCID: PMC6362838.
48. Palesch D, Bosinger SE, Tharp GK, Vanderford TH, Paiardini M, Chahroudi A, Johnson ZP, Kirchhoff F, Hahn BH, Norgren RB, Patel NB, Sodora DL, Dawoud RA, Stewart CB, Seepo SM, Harris RA, Liu Y, Raveendran M, Han Y, English A, Thomas GWC, Hahn MW, Pipes L, Mason CE, Muzny DM, Gibbs RA, Sauter D, Worley K, Rogers J, Silvestri G. Sooty mangabey genome sequence provides insight into AIDS resistance in a natural SIV host. Nature. 2018;553(7686):77-81. doi: 10.1038/nature25140. PubMed PMID: 29300007; PMCID: PMC5843367.
49. Huot N, Rascle P, Petitdemange C, Contreras V, Sturzel CM, Baquero E, Harper JL, Passaes C, Legendre R, Varet H, Madec Y, Sauermann U, Stahl-Hennig C, Nattermann J, Saez-Cirion A, Le Grand R, Keith Reeves R, Paiardini M, Kirchhoff F, Jacquelin B, Muller-Trutwin M. SIV-induced terminally differentiated adaptive NK cells in lymph nodes associated with enhanced MHC-E restricted activity. Nat Commun. 2021;12(1):1282. Epub 20210224. doi: 10.1038/s41467-021-21402-1. PubMed PMID: 33627642; PMCID: PMC7904927.
50. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M, Jr., Estes JD, Lifson JD, Picker LJ. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132-9. Epub 20150119. doi: 10.1038/nm.3781. PubMed PMID: 25599132; PMCID: PMC4320022.
51. Vesterbacka J, Rivera J, Noyan K, Parera M, Neogi U, Calle M, Paredes R, Sonnerborg A, Noguera-Julian M, Nowak P. Richer gut microbiota with distinct metabolic profile in HIV infected Elite Controllers. Sci Rep. 2017;7(1):6269. Epub 20170724. doi: 10.1038/s41598-017-06675-1. PubMed PMID: 28740260; PMCID: PMC5524949.
52. Angin M, Volant S, Passaes C, Lecuroux C, Monceaux V, Dillies MA, Valle-Casuso JC, Pancino G, Vaslin B, Le Grand R, Weiss L, Goujard C, Meyer L, Boufassa F, Muller-Trutwin M, Lambotte O, Saez-Cirion A. Metabolic plasticity of HIV-specific CD8(+) T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat Metab. 2019;1(7):704-16. Epub 20190712. doi: 10.1038/s42255-019-0081-4. PubMed PMID: 32694646.
53. Sekine T, Perez-Potti A, Nguyen S, Gorin JB, Wu VH, Gostick E, Llewellyn-Lacey S, Hammer Q, Falck-Jones S, Vangeti S, Yu M, Smed-Sorensen A, Gaballa A, Uhlin M, Sandberg JK, Brander C, Nowak P, Goepfert PA, Price DA, Betts MR, Buggert M. TOX is expressed by exhausted and polyfunctional human effector memory CD8(+) T cells. Sci Immunol. 2020;5(49). doi: 10.1126/sciimmunol.aba7918. PubMed PMID: 32620560.
54. Jiang C, Lian X, Gao C, Sun X, Einkauf KB, Chevalier JM, Chen SMY, Hua S, Rhee B, Chang K, Blackmer JE, Osborn M, Peluso MJ, Hoh R, Somsouk M, Milush J, Bertagnolli LN, Sweet SE, Varriale JA, Burbelo PD, Chun TW, Laird GM, Serrao E, Engelman AN, Carrington M, Siliciano RF, Siliciano JM, Deeks SG, Walker BD, Lichterfeld M, Yu XG. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature. 2020;585(7824):261-7. Epub 20200826. doi: 10.1038/s41586-020-2651-8. PubMed PMID: 32848246; PMCID: PMC7837306.
55. Lian X, Gao C, Sun X, Jiang C, Einkauf KB, Seiger KW, Chevalier JM, Yuki Y, Martin M, Hoh R, Peluso MJ, Carrington M, Ruiz-Mateos E, Deeks SG, Rosenberg ES, Walker BD, Lichterfeld M, Yu XG. Signatures of immune selection in intact and defective proviruses distinguish HIV-1 elite controllers. Sci Transl Med. 2021;13(624):eabl4097. Epub 20211215. doi: 10.1126/scitranslmed.abl4097. PubMed PMID: 34910552; PMCID: PMC9202005.
56. Perdomo-Celis F, Passaes C, Monceaux V, Volant S, Boufassa F, de Truchis P, Marcou M, Bourdic K, Weiss L, Jung C, Bourgeois C, Goujard C, Meyer L, Muller-Trutwin M, Lambotte O, Saez-Cirion A. Reprogramming dysfunctional CD8+ T cells to promote properties associated with natural HIV control. J Clin Invest. 2022;132(11). doi: 10.1172/JCI157549. PubMed PMID: 35380989; PMCID: PMC9151687.
57. Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, Group AVS. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. Epub 20130314. doi: 10.1371/journal.ppat.1003211. PubMed PMID: 23516360; PMCID: PMC3597518.
58. Galvez C, Urrea V, Dalmau J, Jimenez M, Clotet B, Monceaux V, Huot N, Leal L, Gonzalez-Soler V, Gonzalez-Cao M, Muller-Trutwin M, Saez-Cirion A, Garcia F, Blanco J, Martinez-Picado J, Salgado M. Extremely low viral reservoir in treated chronically HIV-1-infected individuals. EBioMedicine. 2020;57:102830. Epub 20200621. doi: 10.1016/j.ebiom.2020.102830. PubMed PMID: 32580136; PMCID: PMC7317241.
59. Strongin Z, Micci L, Fromentin R, Harper J, McBrien J, Ryan E, Shenvi N, Easley K, Chomont N, Silvestri G, Paiardini M. Virologic and Immunologic Features of Simian Immunodeficiency Virus Control Post-ART Interruption in Rhesus Macaques. J Virol. 2020;94(14). Epub 20200701. doi: 10.1128/JVI.00338-20. PubMed PMID: 32350073; PMCID: PMC7343203.
60. Molinos-Albert LM, Lorin V, Monceaux V, Orr S, Essat A, Dufloo J, Schwartz O, Rouzioux C, Meyer L, Hocqueloux L, Saez-Cirion A, Mouquet H, Group AVS. Transient viral exposure drives functionally-coordinated humoral immune responses in HIV-1 post-treatment controllers. Nat Commun. 2022;13(1):1944. Epub 20220411. doi: 10.1038/s41467-022-29511-1. PubMed PMID: 35410989; PMCID: PMC9001681.
61. Galvez C, Urrea V, Garcia-Guerrero MDC, Bernal S, Benet S, Mothe B, Bailon L, Dalmau J, Martinez A, Nieto A, Leal L, Garcia F, Clotet B, Martinez-Picado J, Salgado M. Altered T-cell subset distribution in the viral reservoir in HIV-1-infected individuals with extremely low proviral DNA (LoViReTs). J Intern Med. 2022;292(2):308-20. Epub 20220328. doi: 10.1111/joim.13484. PubMed PMID: 35342993; PMCID: PMC9308636.
62. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350-4. Epub 20060820. doi: 10.1038/nature05115. PubMed PMID: 16921384.
63. McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, Kuri-Cervantes L, Benne C, Ryan ES, Balderas R, Jean S, Easley K, Marconi V, Silvestri G, Estes JD, Sekaly RP, Paiardini M. CTLA-4(+)PD-1(-) Memory CD4(+) T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity. 2017;47(4):776-88 e5. doi: 10.1016/j.immuni.2017.09.018. PubMed PMID: 29045906; PMCID: PMC5679306.
64. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, Perreau M. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. 2016;22(7):754-61. Epub 20160530. doi: 10.1038/nm.4113. PubMed PMID: 27239760.
65. Ren Y, Huang SH, Patel S, Alberto WDC, Magat D, Ahimovic D, Macedo AB, Durga R, Chan D, Zale E, Mota TM, Truong R, Rohwetter T, McCann CD, Kovacs CM, Benko E, Wimpelberg A, Cannon C, Hardy WD, Bosque A, Bollard CM, Jones RB. BCL-2 antagonism sensitizes cytotoxic T cell-resistant HIV reservoirs to elimination ex vivo. J Clin Invest. 2020;130(5):2542-59. doi: 10.1172/JCI132374. PubMed PMID: 32027622; PMCID: PMC7191002.
66. Harper J, Ribeiro SP, Chan CN, Aid M, Deleage C, Micci L, Pino M, Cervasi B, Raghunathan G, Rimmer E, Ayanoglu G, Wu G, Shenvi N, Barnard RJ, Del Prete GQ, Busman-Sahay K, Silvestri G, Kulpa DA, Bosinger SE, Easley KA, Howell BJ, Gorman D, Hazuda DJ, Estes JD, Sekaly RP, Paiardini M. Interleukin-10 contributes to reservoir establishment and persistence in SIV-infected macaques treated with antiretroviral therapy. J Clin Invest. 2022;132(8). doi: 10.1172/JCI155251. PubMed PMID: 35230978; PMCID: PMC9012284.
67. Mutascio S, Mota T, Franchitti L, Sharma AA, Willemse A, Bergstresser SN, Wang H, Statzu M, Tharp GK, Weiler J, Sekaly RP, Bosinger SE, Paiardini M, Silvestri G, Jones RB, Kulpa DA. CD8(+) T cells promote HIV latency by remodeling CD4(+) T cell metabolism to enhance their survival, quiescence, and stemness. Immunity. 2023;56(5):1132-47 e6. Epub 20230407. doi: 10.1016/j.immuni.2023.03.010. PubMed PMID: 37030290.
68. Honeycutt JB, Wahl A, Baker C, Spagnuolo RA, Foster J, Zakharova O, Wietgrefe S, Caro-Vegas C, Madden V, Sharpe G, Haase AT, Eron JJ, Garcia JV. Macrophages sustain HIV replication in vivo independently of T cells. J Clin Invest. 2016;126(4):1353-66. Epub 20160307. doi: 10.1172/JCI84456. PubMed PMID: 26950420; PMCID: PMC4811134.
69. Descours B, Petitjean G, Lopez-Zaragoza JL, Bruel T, Raffel R, Psomas C, Reynes J, Lacabaratz C, Levy Y, Schwartz O, Lelievre JD, Benkirane M. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature. 2017;543(7646):564-7. Epub 20170315. doi: 10.1038/nature21710. PubMed PMID: 28297712.
70. Bertagnolli LN, White JA, Simonetti FR, Beg SA, Lai J, Tomescu C, Murray AJ, Antar AAR, Zhang H, Margolick JB, Hoh R, Deeks SG, Tebas P, Montaner LJ, Siliciano RF, Laird GM, Siliciano JD. The role of CD32 during HIV-1 infection. Nature. 2018;561(7723):E17-E9. Epub 20180919. doi: 10.1038/s41586-018-0494-3. PubMed PMID: 30232425; PMCID: PMC6442722.
71. Perez L, Anderson J, Chipman J, Thorkelson A, Chun TW, Moir S, Haase AT, Douek DC, Schacker TW, Boritz EA. Conflicting evidence for HIV enrichment in CD32(+) CD4 T cells. Nature. 2018;561(7723):E9-E16. Epub 20180919. doi: 10.1038/s41586-018-0493-4. PubMed PMID: 30232423; PMCID: PMC6410373.
72. Osuna CE, Lim SY, Kublin JL, Apps R, Chen E, Mota TM, Huang SH, Ren Y, Bachtel ND, Tsibris AM, Ackerman ME, Jones RB, Nixon DF, Whitney JB. Evidence that CD32a does not mark the HIV-1 latent reservoir. Nature. 2018;561(7723):E20-E8. Epub 20180919. doi: 10.1038/s41586-018-0495-2. PubMed PMID: 30232424; PMCID: PMC6528470.
73. Clark IC, Mudvari P, Thaploo S, Smith S, Abu-Laban M, Hamouda M, Theberge M, Shah S, Ko SH, Perez L, Bunis DG, Lee JS, Kilam D, Zakaria S, Choi S, Darko S, Henry AR, Wheeler MA, Hoh R, Butrus S, Deeks SG, Quintana FJ, Douek DC, Abate AR, Boritz EA. HIV silencing and cell survival signatures in infected T cell reservoirs. Nature. 2023;614(7947):318-25. Epub 20230104. doi: 10.1038/s41586-022-05556-6. PubMed PMID: 36599978; PMCID: PMC9908556.
74. Sun W, Gao C, Hartana CA, Osborn MR, Einkauf KB, Lian X, Bone B, Bonheur N, Chun TW, Rosenberg ES, Walker BD, Yu XG, Lichterfeld M. Phenotypic signatures of immune selection in HIV-1 reservoir cells. Nature. 2023;614(7947):309-17. Epub 20230104. doi: 10.1038/s41586-022-05538-8. PubMed PMID: 36599977; PMCID: PMC9908552.
75. Swanstrom AE, Immonen TT, Oswald K, Pyle C, Thomas JA, Bosche WJ, Silipino L, Hull M, Newman L, Coalter V, Wiles A, Wiles R, Kiser J, Morcock DR, Shoemaker R, Fast R, Breed MW, Kramer J, Donohue D, Malys T, Fennessey CM, Trubey CM, Deleage C, Estes JD, Lifson JD, Keele BF, Del Prete GQ. Antibody-mediated depletion of viral reservoirs is limited in SIV-infected macaques treated early with antiretroviral therapy. J Clin Invest. 2021;131(6). doi: 10.1172/JCI142421. PubMed PMID: 33465055; PMCID: PMC7954603.
76. Vennhuis RT, editor. CD4+ depletion results in robust CNS rebound and larger macrophage reservoirs. CROI; 2022 February 16; Virtual.
77. Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis. 2014;27(1):29-35. doi: 10.1097/QCO.0000000000000026. PubMed PMID: 24296585; PMCID: PMC4031321.
78. Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482-5. Epub 20120725. doi: 10.1038/nature11286. PubMed PMID: 22837004; PMCID: PMC3704185.
79. Miller JS, Davis ZB, Helgeson E, Reilly C, Thorkelson A, Anderson J, Lima NS, Jorstad S, Hart GT, Lee JH, Safrit JT, Wong H, Cooley S, Gharu L, Chung H, Soon-Shiong P, Dobrowolski C, Fletcher CV, Karn J, Douek DC, Schacker TW. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial. Nat Med. 2022;28(2):392-400. Epub 20220131. doi: 10.1038/s41591-021-01651-9. PubMed PMID: 35102335.
80. McBrien JB, Mavigner M, Franchitti L, Smith SA, White E, Tharp GK, Walum H, Busman-Sahay K, Aguilera-Sandoval CR, Thayer WO, Spagnuolo RA, Kovarova M, Wahl A, Cervasi B, Margolis DM, Vanderford TH, Carnathan DG, Paiardini M, Lifson JD, Lee JH, Safrit JT, Bosinger SE, Estes JD, Derdeyn CA, Garcia JV, Kulpa DA, Chahroudi A, Silvestri G. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8(+) cells. Nature. 2020;578(7793):154-9. Epub 20200122. doi: 10.1038/s41586-020-1946-0. PubMed PMID: 31969705; PMCID: PMC7580846.
81. Harper J, Gordon S, Chan CN, Wang H, Lindemuth E, Galardi C, Falcinelli SD, Raines SLM, Read JL, Nguyen K, McGary CS, Nekorchuk M, Busman-Sahay K, Schawalder J, King C, Pino M, Micci L, Cervasi B, Jean S, Sanderson A, Johns B, Koblansky AA, Amrine-Madsen H, Lifson J, Margolis DM, Silvestri G, Bar KJ, Favre D, Estes JD, Paiardini M. CTLA-4 and PD-1 dual blockade induces SIV reactivation without control of rebound after antiretroviral therapy interruption. Nat Med. 2020;26(4):519-28. Epub 20200316. doi: 10.1038/s41591-020-0782-y. PubMed PMID: 32284611; PMCID: PMC7790171.
82. Rahman SA, Yagnik B, Bally AP, Morrow KN, Wang S, Vanderford TH, Freeman GJ, Ahmed R, Amara RR. PD-1 blockade and vaccination provide therapeutic benefit against SIV by inducing broad and functional CD8(+) T cells in lymphoid tissue. Sci Immunol. 2021;6(63):eabh3034. Epub 20210903. doi: 10.1126/sciimmunol.abh3034. PubMed PMID: 34516743; PMCID: PMC8500359.
83. Nixon CC, Mavigner M, Sampey GC, Brooks AD, Spagnuolo RA, Irlbeck DM, Mattingly C, Ho PT, Schoof N, Cammon CG, Tharp GK, Kanke M, Wang Z, Cleary RA, Upadhyay AA, De C, Wills SR, Falcinelli SD, Galardi C, Walum H, Schramm NJ, Deutsch J, Lifson JD, Fennessey CM, Keele BF, Jean S, Maguire S, Liao B, Browne EP, Ferris RG, Brehm JH, Favre D, Vanderford TH, Bosinger SE, Jones CD, Routy JP, Archin NM, Margolis DM, Wahl A, Dunham RM, Silvestri G, Chahroudi A, Garcia JV. Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature. 2020;578(7793):160-5. Epub 20200122. doi: 10.1038/s41586-020-1951-3. PubMed PMID: 31969707; PMCID: PMC7111210.
84. Dashti A, Sukkestad S, Horner AM, Neja M, Siddiqi Z, Waller C, Goldy J, Monroe D, Lin A, Schoof N, Singh V, Mavigner M, Lifson JD, Deleage C, Tuyishime M, Falcinelli SD, King HAD, Ke R, Mason RD, Archin NM, Dunham RM, Safrit JT, Jean S, Perelson AS, Margolis DM, Ferrari G, Roederer M, Silvestri G, Chahroudi A. AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques. Nat Med. 2023. Epub 20231002. doi: 10.1038/s41591-023-02570-7. PubMed PMID: 37783968.
85. Lim SY, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, Miller MD, Cihlar T, Lee WA, Hill AL, Whitney JB. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med. 2018;10(439). doi: 10.1126/scitranslmed.aao4521. PubMed PMID: 29720451; PMCID: PMC5973480.
86. Bekerman E, Hesselgesser J, Carr B, Nagel M, Hung M, Wang A, Stapleton L, von Gegerfelt A, Elyard HA, Lifson JD, Geleziunas R. PD-1 Blockade and TLR7 Activation Lack Therapeutic Benefit in Chronic Simian Immunodeficiency Virus-Infected Macaques on Antiretroviral Therapy. Antimicrob Agents Chemother. 2019;63(11). Epub 20191022. doi: 10.1128/AAC.01163-19. PubMed PMID: 31501143; PMCID: PMC6811450.
87. Del Prete GQ, Alvord WG, Li Y, Deleage C, Nag M, Oswald K, Thomas JA, Pyle C, Bosche WJ, Coalter V, Wiles A, Wiles R, Berkemeier B, Hull M, Chipriano E, Silipino L, Fast R, Kiser J, Kiser R, Malys T, Kramer J, Breed MW, Trubey CM, Estes JD, Barnes TL, Hesselgesser J, Geleziunas R, Lifson JD. TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight. 2019;4(11). Epub 20190606. doi: 10.1172/jci.insight.127717. PubMed PMID: 31167974; PMCID: PMC6629134.
88. Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540-51. Epub 20131024. doi: 10.1016/j.cell.2013.09.020. PubMed PMID: 24243014; PMCID: PMC3896327.
89. Siliciano JD, Siliciano RF. Low Inducibility of Latent Human Immunodeficiency Virus Type 1 Proviruses as a Major Barrier to Cure. J Infect Dis. 2021;223(12 Suppl 2):13-21. doi: 10.1093/infdis/jiaa649. PubMed PMID: 33586775; PMCID: PMC7883034.
90. Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319-27. doi: 10.7326/M14-1027. PubMed PMID: 25047577; PMCID: PMC4236912.
91. Vansant G, Bruggemans A, Janssens J, Debyser Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses. 2020;12(1). Epub 20200110. doi: 10.3390/v12010084. PubMed PMID: 31936859; PMCID: PMC7019976.
92. Sekaly R, Ribeiro SP, Strongin Z, Caten FT, Ghneim K, Sanchez GP, de Medeiros GX, Pelletier A-N, Hoang T, Nguyen K, Jean S, Wallace C, Balderas R, Lifson L, Raghunathan G, Rimmer E, Pastuskovas C, Wu G, Micci L, Ribeiro R, Chan C, Estes J, Silvestri G, Gorman DM, Bonnie B, Hazuda DJ, Paiardini M. Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption. Research Square. 2023. doi: 10.21203/rs.3.rs-3175716/v1.
93. NIAID. Experimental HIV vaccine regimen safe but ineffective, study finds [updated 2023 January 18; cited 2023 October 4]. Available from: https://www.nih.gov/news-events/news-releases/experimental-hiv-vaccine-regimen-safe-ineffective-study-finds.
94. HVTN. Phase 3 Mosaic-based investigational HIV vaccine study discontinued following disappointing results of planned data review [updated 2023 January 18; cited 2023 October 4]. Available from: https://www.hvtn.org/news/news-releases/2023/01/phase-3-mosaic-based-investigational-hiv-vaccine-study-discontinued-following-disappointing-results-planned-data-review.html.
95. Gray GE, Bekker LG, Laher F, Malahleha M, Allen M, Moodie Z, Grunenberg N, Huang Y, Grove D, Prigmore B, Kee JJ, Benkeser D, Hural J, Innes C, Lazarus E, Meintjes G, Naicker N, Kalonji D, Nchabeleng M, Sebe M, Singh N, Kotze P, Kassim S, Dubula T, Naicker V, Brumskine W, Ncayiya CN, Ward AM, Garrett N, Kistnasami G, Gaffoor Z, Selepe P, Makhoba PB, Mathebula MP, Mda P, Adonis T, Mapetla KS, Modibedi B, Philip T, Kobane G, Bentley C, Ramirez S, Takuva S, Jones M, Sikhosana M, Atujuna M, Andrasik M, Hejazi NS, Puren A, Wiesner L, Phogat S, Diaz Granados C, Koutsoukos M, Van Der Meeren O, Barnett SW, Kanesa-Thasan N, Kublin JG, McElrath MJ, Gilbert PB, Janes H, Corey L, Team HS. Vaccine Efficacy of ALVAC-HIV and Bivalent Subtype C gp120-MF59 in Adults. N Engl J Med. 2021;384(12):1089-100. doi: 10.1056/NEJMoa2031499. PubMed PMID: 33761206; PMCID: PMC7888373.
96. HVTN. Experimental phase 2B HIV vaccine regimen provides insufficient protection in preventing HIV [updated 2021 August 31; cited 2023 October 4]. Available from: https://www.hvtn.org/news/news-releases/2021/08/experimental-phase-2b-hiv-vaccine-regimen-provides-insufficient-.html.
97. Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O’Dell SJ, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, Chun TW. Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med. 2016;375(21):2037-50. Epub 20161109. doi: 10.1056/NEJMoa1608243. PubMed PMID: 27959728; PMCID: PMC5292134.
98. Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O’Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, Team VRCS. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206. doi: 10.1126/scitranslmed.aad5752. PubMed PMID: 26702094.
99. Crowell TA, Colby DJ, Pinyakorn S, Sacdalan C, Pagliuzza A, Intasan J, Benjapornpong K, Tangnaree K, Chomchey N, Kroon E, de Souza MS, Tovanabutra S, Rolland M, Eller MA, Paquin-Proulx D, Bolton DL, Tokarev A, Thomas R, Takata H, Trautmann L, Krebs SJ, Modjarrad K, McDermott AB, Bailer RT, Doria-Rose N, Patel B, Gorelick RJ, Fullmer BA, Schuetz A, Grandin PV, O’Connell RJ, Ledgerwood JE, Graham BS, Tressler R, Mascola JR, Chomont N, Michael NL, Robb ML, Phanuphak N, Ananworanich J, Group RVS. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV. 2019;6(5):e297-e306. Epub 20190415. doi: 10.1016/S2352-3018(19)30053-0. PubMed PMID: 31000477; PMCID: PMC6693657.
100. Stephenson KE, Julg B, Tan CS, Zash R, Walsh SR, Rolle CP, Monczor AN, Lupo S, Gelderblom HC, Ansel JL, Kanjilal DG, Maxfield LF, Nkolola J, Borducchi EN, Abbink P, Liu J, Peter L, Chandrashekar A, Nityanandam R, Lin Z, Setaro A, Sapiente J, Chen Z, Sunner L, Cassidy T, Bennett C, Sato A, Mayer B, Perelson AS, deCamp A, Priddy FH, Wagh K, Giorgi EE, Yates NL, Arduino RC, DeJesus E, Tomaras GD, Seaman MS, Korber B, Barouch DH. Safety, pharmacokinetics and antiviral activity of PGT121, a broadly neutralizing monoclonal antibody against HIV-1: a randomized, placebo-controlled, phase 1 clinical trial. Nat Med. 2021;27(10):1718-24. Epub 20211007. doi: 10.1038/s41591-021-01509-0. PubMed PMID: 34621054; PMCID: PMC8516645.
101. Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP, Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487-91. Epub 20150408. doi: 10.1038/nature14411. PubMed PMID: 25855300; PMCID: PMC4890714.
102. Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP, Jr., Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC, Caskey M. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556-60. Epub 20160622. doi: 10.1038/nature18929. PubMed PMID: 27338952; PMCID: PMC5034582.
103. Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, Learn GH, Cohen YZ, Lehmann C, Gillor D, Shimeliovich I, Unson-O’Brien C, Weiland D, Robles A, Kummerle T, Wyen C, Levin R, Witmer-Pack M, Eren K, Ignacio C, Kiss S, West AP, Jr., Mouquet H, Zingman BS, Gulick RM, Keler T, Bjorkman PJ, Seaman MS, Hahn BH, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC, Klein F. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185-91. Epub 20170116. doi: 10.1038/nm.4268. PubMed PMID: 28092665; PMCID: PMC5467219.
104. Wagh K, Seaman MS. Divide and conquer: broadly neutralizing antibody combinations for improved HIV-1 viral coverage. Curr Opin HIV AIDS. 2023;18(4):164-70. Epub 20230519. doi: 10.1097/COH.0000000000000800. PubMed PMID: 37249911; PMCID: PMC10256304.
105. Julg B, Stephenson KE, Wagh K, Tan SC, Zash R, Walsh S, Ansel J, Kanjilal D, Nkolola J, Walker-Sperling VEK, Ophel J, Yanosick K, Borducchi EN, Maxfield L, Abbink P, Peter L, Yates NL, Wesley MS, Hassell T, Gelderblom HC, deCamp A, Mayer BT, Sato A, Gerber MW, Giorgi EE, Gama L, Koup RA, Mascola JR, Monczor A, Lupo S, Rolle CP, Arduino R, DeJesus E, Tomaras GD, Seaman MS, Korber B, Barouch DH. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nat Med. 2022;28(6):1288-96. Epub 20220512. doi: 10.1038/s41591-022-01815-1. PubMed PMID: 35551291; PMCID: PMC9205771.
106. Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, Cohen YZ, Wyen C, Kummerle T, Karagounis T, Lu CL, Handl L, Unson-O’Brien C, Patel R, Ruping C, Schlotz M, Witmer-Pack M, Shimeliovich I, Kremer G, Thomas E, Seaton KE, Horowitz J, West AP, Jr., Bjorkman PJ, Tomaras GD, Gulick RM, Pfeifer N, Fatkenheuer G, Seaman MS, Klein F, Caskey M, Nussenzweig MC. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479-84. Epub 20180926. doi: 10.1038/s41586-018-0531-2. PubMed PMID: 30258136; PMCID: PMC6166473.
107. Sneller MC, Blazkova J, Justement JS, Shi V, Kennedy BD, Gittens K, Tolstenko J, McCormack G, Whitehead EJ, Schneck RF, Proschan MA, Benko E, Kovacs C, Oguz C, Seaman MS, Caskey M, Nussenzweig MC, Fauci AS, Moir S, Chun TW. Combination anti-HIV antibodies provide sustained virological suppression. Nature. 2022;606(7913):375-81. Epub 20220601. doi: 10.1038/s41586-022-04797-9. PubMed PMID: 35650437.
108. Gaebler C, Nogueira L, Stoffel E, Oliveira TY, Breton G, Millard KG, Turroja M, Butler A, Ramos V, Seaman MS, Reeves JD, Petroupoulos CJ, Shimeliovich I, Gazumyan A, Jiang CS, Jilg N, Scheid JF, Gandhi R, Walker BD, Sneller MC, Fauci A, Chun TW, Caskey M, Nussenzweig MC. Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature. 2022;606(7913):368-74. Epub 20220413. doi: 10.1038/s41586-022-04597-1. PubMed PMID: 35418681; PMCID: PMC9177424.
109. Parikh UM, Bacheler L, Koontz D, Mellors JW. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J Virol. 2006;80(10):4971-7. doi: 10.1128/JVI.80.10.4971-4977.2006. PubMed PMID: 16641288; PMCID: PMC1472090.
110. Niessl J, Baxter AE, Mendoza P, Jankovic M, Cohen YZ, Butler AL, Lu CL, Dube M, Shimeliovich I, Gruell H, Klein F, Caskey M, Nussenzweig MC, Kaufmann DE. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med. 2020;26(2):222-7. Epub 20200203. doi: 10.1038/s41591-019-0747-1. PubMed PMID: 32015556; PMCID: PMC7018622.
111. Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, Donau OK, Plishka RJ, Buckler-White A, Seaman MS, Lifson JD, Koup RA, Fauci AS, Nussenzweig MC, Martin MA. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543(7646):559-63. Epub 20170313. doi: 10.1038/nature21435. PubMed PMID: 28289286; PMCID: PMC5458531.
112. Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86(3):367-77. doi: 10.1016/s0092-8674(00)80110-5. PubMed PMID: 8756719.
113. Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692-8. doi: 10.1056/NEJMoa0802905. PubMed PMID: 19213682.
114. Yukl SA, Boritz E, Busch M, Bentsen C, Chun TW, Douek D, Eisele E, Haase A, Ho YC, Hutter G, Justement JS, Keating S, Lee TH, Li P, Murray D, Palmer S, Pilcher C, Pillai S, Price RW, Rothenberger M, Schacker T, Siliciano J, Siliciano R, Sinclair E, Strain M, Wong J, Richman D, Deeks SG. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9(5):e1003347. Epub 20130509. doi: 10.1371/journal.ppat.1003347. PubMed PMID: 23671416; PMCID: PMC3649997.
115. Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes AJ, Muir L, Waters L, Frater J, Lever AML, Edwards SG, Gabriel IH, Olavarria E. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568(7751):244-8. Epub 20190305. doi: 10.1038/s41586-019-1027-4. PubMed PMID: 30836379; PMCID: PMC7275870.
116. Gupta RK, Peppa D, Hill AL, Galvez C, Salgado M, Pace M, McCoy LE, Griffith SA, Thornhill J, Alrubayyi A, Huyveneers LEP, Nastouli E, Grant P, Edwards SG, Innes AJ, Frater J, Nijhuis M, Wensing AMJ, Martinez-Picado J, Olavarria E. Evidence for HIV-1 cure after CCR5Delta32/Delta32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV. 2020;7(5):e340-e7. Epub 20200310. doi: 10.1016/S2352-3018(20)30069-2. PubMed PMID: 32169158; PMCID: PMC7606918.
117. Hsu J, Van Besien K, Glesby MJ, Pahwa S, Coletti A, Warshaw MG, Petz L, Moore TB, Chen YH, Pallikkuth S, Dhummakupt A, Cortado R, Golner A, Bone F, Baldo M, Riches M, Mellors JW, Tobin NH, Browning R, Persaud D, Bryson Y, International Maternal Pediatric Adolescent ACTNPT. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell. 2023;186(6):1115-26 e8. doi: 10.1016/j.cell.2023.02.030. PubMed PMID: 36931242.
118. Jensen BO, Knops E, Cords L, Lubke N, Salgado M, Busman-Sahay K, Estes JD, Huyveneers LEP, Perdomo-Celis F, Wittner M, Galvez C, Mummert C, Passaes C, Eberhard JM, Munk C, Hauber I, Hauber J, Heger E, De Clercq J, Vandekerckhove L, Bergmann S, Dunay GA, Klein F, Haussinger D, Fischer JC, Nachtkamp K, Timm J, Kaiser R, Harrer T, Luedde T, Nijhuis M, Saez-Cirion A, Schulze Zur Wiesch J, Wensing AMJ, Martinez-Picado J, Kobbe G. In-depth virological and immunological characterization of HIV-1 cure after CCR5Delta32/Delta32 allogeneic hematopoietic stem cell transplantation. Nat Med. 2023;29(3):583-7. Epub 20230220. doi: 10.1038/s41591-023-02213-x. PubMed PMID: 36807684; PMCID: PMC10033413.
119. Dickter J, Weibel S, Cardoso A, Li S, Gendzekhadze K, Feng Y, Dadwal S, Taplitz R, Ross J, Aribi A, Stan R, Kidambi T, Lai L, Chang S, Chaillon A, Al Malki M, Alvarnas J, Forman S, Zaia J, editors. The ‘City of Hope’ Patient: prolonged HIV-1 remission without antiretrovirals (ART) after allogeneic hematopoietic stem cell transplantation (aHCT) of CCR5-Delta32/Delta32 donor cells for acute myelogenous leukemia (AML). AIDS; 2022 August 1; Montreal, Canada.
120. Rothenberger M, Wagner JE, Haase A, Richman D, Grzywacz B, Strain M, Lada S, Estes J, Fletcher CV, Podany AT, Anderson J, Schmidt T, Wietgrefe S, Schacker T, Verneris MR. Transplantation of CCR5∆32 Homozygous Umbilical Cord Blood in a Child With Acute Lymphoblastic Leukemia and Perinatally Acquired HIV Infection. Open Forum Infect Dis. 2018;5(5):ofy090. Epub 20180522. doi: 10.1093/ofid/ofy090. PubMed PMID: 29868623; PMCID: PMC5965100.
121. Duarte RF, Salgado M, Sanchez-Ortega I, Arnan M, Canals C, Domingo-Domenech E, Fernandez-de-Sevilla A, Gonzalez-Barca E, Moron-Lopez S, Nogues N, Patino B, Puertas MC, Clotet B, Petz LD, Querol S, Martinez-Picado J. CCR5 Delta32 homozygous cord blood allogeneic transplantation in a patient with HIV: a case report. Lancet HIV. 2015;2(6):e236-42. Epub 20150519. doi: 10.1016/S2352-3018(15)00083-1. PubMed PMID: 26423196.
122. Huyveneers LEP, Bruns A, Stam A, Ellerbroek P, de Jong D, Nagy NA, Gumbs SBH, Tesselaar K, Bosman K, Salgado M, Hutter G, Brosens LAA, Kwon M, Diez Martin J, van der Meer JTM, de Kort TM, Saez-Cirion A, Schulze Zur Wiesch J, Boelens JJ, Martinez-Picado J, Kuball JHE, Wensing AMJ, Nijhuis M, IciStem C. Autopsy Study Defines Composition and Dynamics of the HIV-1 Reservoir after Allogeneic Hematopoietic Stem Cell Transplantation with CCR5Delta32/Delta32 Donor Cells. Viruses. 2022;14(9). Epub 20220917. doi: 10.3390/v14092069. PubMed PMID: 36146874; PMCID: PMC9503691.
123. Hutter G. More on shift of HIV tropism in stem-cell transplantation with CCR5 delta32/delta32 mutation. N Engl J Med. 2014;371(25):2437-8. doi: 10.1056/NEJMc1412279. PubMed PMID: 25517721.
124. Kordelas L, Verheyen J, Beelen DW, Horn PA, Heinold A, Kaiser R, Trenschel R, Schadendorf D, Dittmer U, Esser S, Essen HIVAG. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med. 2014;371(9):880-2. doi: 10.1056/NEJMc1405805. PubMed PMID: 25162903.
125. Jubb B, Lewis M, McFadyen L, Simpson P, Mori J, Chan P, Weatherley B, van der Ryst E, Westby M, Craig C. Incidence of CXCR4 tropism and CCR5-tropic resistance in treatment-experienced participants receiving maraviroc in the 48-week MOTIVATE 1 and 2 trials. Antivir Chem Chemother. 2019;27:2040206619895706. doi: 10.1177/2040206619895706. PubMed PMID: 31856576; PMCID: PMC6931239.
126. Cillo AR, Krishnan S, McMahon DK, Mitsuyasu RT, Para MF, Mellors JW. Impact of chemotherapy for HIV-1 related lymphoma on residual viremia and cellular HIV-1 DNA in patients on suppressive antiretroviral therapy. PLoS One. 2014;9(3):e92118. Epub 20140317. doi: 10.1371/journal.pone.0092118. PubMed PMID: 24638072; PMCID: PMC3956871.
127. Mavigner M, Watkins B, Lawson B, Lee ST, Chahroudi A, Kean L, Silvestri G. Persistence of virus reservoirs in ART-treated SHIV-infected rhesus macaques after autologous hematopoietic stem cell transplant. PLoS Pathog. 2014;10(9):e1004406. Epub 20140925. doi: 10.1371/journal.ppat.1004406. PubMed PMID: 25254512; PMCID: PMC4177994.
128. Peterson CW, Benne C, Polacino P, Kaur J, McAllister CE, Filali-Mouhim A, Obenza W, Pecor TA, Huang ML, Baldessari A, Murnane RD, Woolfrey AE, Jerome KR, Hu SL, Klatt NR, DeRosa S, Sekaly RP, Kiem HP. Loss of immune homeostasis dictates SHIV rebound after stem-cell transplantation. JCI Insight. 2017;2(4):e91230. Epub 20170223. doi: 10.1172/jci.insight.91230. PubMed PMID: 28239658; PMCID: PMC5322807.
129. Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997;16(1):100-3. doi: 10.1038/ng0597-100. PubMed PMID: 9140404.
130. Solloch UV, Lang K, Lange V, Bohme I, Schmidt AH, Sauter J. Frequencies of gene variant CCR5-Delta32 in 87 countries based on next-generation sequencing of 1.3 million individuals sampled from 3 national DKMS donor centers. Hum Immunol. 2017;78(11-12):710-7. Epub 20171005. doi: 10.1016/j.humimm.2017.10.001. PubMed PMID: 28987960.
131. Dash PK, Chen C, Kaminski R, Su H, Mancuso P, Sillman B, Zhang C, Liao S, Sravanam S, Liu H, Waight E, Guo L, Mathews S, Sariyer R, Mosley RL, Poluektova LY, Caocci M, Amini S, Gorantla S, Burdo TH, Edagwa B, Gendelman HE, Khalili K. CRISPR editing of CCR5 and HIV-1 facilitates viral elimination in antiretroviral drug-suppressed virus-infected humanized mice. Proc Natl Acad Sci U S A. 2023;120(19):e2217887120. Epub 20230501. doi: 10.1073/pnas.2217887120. PubMed PMID: 37126704; PMCID: PMC10175831.
132. Mancuso P, Chen C, Kaminski R, Gordon J, Liao S, Robinson JA, Smith MD, Liu H, Sariyer IK, Sariyer R, Peterson TA, Donadoni M, Williams JB, Siddiqui S, Bunnell BA, Ling B, MacLean AG, Burdo TH, Khalili K. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat Commun. 2020;11(1):6065. Epub 20201127. doi: 10.1038/s41467-020-19821-7. PubMed PMID: 33247091; PMCID: PMC7695718.
133. Urnov FD, Miller JC, Lee YL, Beausejour CM, Rock JM, Augustus S, Jamieson AC, Porteus MH, Gregory PD, Holmes MC. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435(7042):646-51. Epub 20050403. doi: 10.1038/nature03556. PubMed PMID: 15806097.
134. Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26(7):808-16. Epub 20080629. doi: 10.1038/nbt1410. PubMed PMID: 18587387; PMCID: PMC3422503.
135. Tebas P, Jadlowsky JK, Shaw PA, Tian L, Esparza E, Brennan AL, Kim S, Naing SY, Richardson MW, Vogel AN, Maldini CR, Kong H, Liu X, Lacey SF, Bauer AM, Mampe F, Richman LP, Lee G, Ando D, Levine BL, Porter DL, Zhao Y, Siegel DL, Bar KJ, June CH, Riley JL. CCR5-edited CD4+ T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J Clin Invest. 2021;131(7). doi: 10.1172/JCI144486. PubMed PMID: 33571163; PMCID: PMC8011906.
136. Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, Holmes MC, Gregory PD, Ando DG, Kalos M, Collman RG, Binder-Scholl G, Plesa G, Hwang WT, Levine BL, June CH. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901-10. doi: 10.1056/NEJMoa1300662. PubMed PMID: 24597865; PMCID: PMC4084652.
137. Zeidan J, Sharma AA, Lee G, Raad A, Fromentin R, Fourati S, Ghneim K, Sanchez GP, Benne C, Canderan G, Procopio FA, Balderas R, Monette G, Lalezari JP, Heffernan JM, Sabbagh L, Chomont N, Ando D, Deeks SG, Sekaly R-P. Infusion of CCR5 gene-edited T cells allows immune reconstitution, HIV reservoir decay, and long-term virological control. bioRxiv. 2021. doi: 10.1101/2021.02.28.433290.
138. Henrich TJ, Hu Z, Li JZ, Sciaranghella G, Busch MP, Keating SM, Gallien S, Lin NH, Giguel FF, Lavoie L, Ho VT, Armand P, Soiffer RJ, Sagar M, Lacasce AS, Kuritzkes DR. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207(11):1694-702. Epub 20130304. doi: 10.1093/infdis/jit086. PubMed PMID: 23460751; PMCID: PMC3636784.
139. Cummins NW, Rizza S, Litzow MR, Hua S, Lee GQ, Einkauf K, Chun TW, Rhame F, Baker JV, Busch MP, Chomont N, Dean PG, Fromentin R, Haase AT, Hampton D, Keating SM, Lada SM, Lee TH, Natesampillai S, Richman DD, Schacker TW, Wietgrefe S, Yu XG, Yao JD, Zeuli J, Lichterfeld M, Badley AD. Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study. PLoS Med. 2017;14(11):e1002461. Epub 20171128. doi: 10.1371/journal.pmed.1002461. PubMed PMID: 29182633; PMCID: PMC5705162.
140. Sáez-Cirión A, Mamez A-C, Avettand-Fenoel V, Thoueille P, Nabergoj M, Hentzien M, Mereles Costa E, Salgado M, Nijhuis M, Melard A, Gardiennet E, Monceaux V, Passaes C, Chapel A, Perdomo-Celis F, Wensing A, Martínez-Picado J, Yerly S, Rougemont M, Calmy A, ICISTEM, editors. Absence of viral rebound for 18 months without antiretrovirals after allogeneic hematopoietic stem cell transplantation with wild-type CCR5 donor cells to treat a biphenotypic sarcoma. IAS; 2023 July 16; Brisbane, Australia.
141. Wu HL, Busman-Sahay K, Weber WC, Waytashek CM, Boyle CD, Bateman KB, Reed JS, Hwang JM, Shriver-Munsch C, Swanson T, Northrup M, Armantrout K, Price H, Robertson-LeVay M, Uttke S, Kumar MR, Fray EJ, Taylor-Brill S, Bondoc S, Agnor R, Junell SL, Legasse AW, Moats C, Bochart RM, Sciurba J, Bimber BN, Sullivan MN, Dozier B, MacAllister RP, Hobbs TR, Martin LD, Panoskaltsis-Mortari A, Colgin LMA, Siliciano RF, Siliciano JD, Estes JD, Smedley JV, Axthelm MK, Meyers G, Maziarz RT, Burwitz BJ, Stanton JJ, Sacha JB. Allogeneic immunity clears latent virus following allogeneic stem cell transplantation in SIV-infected ART-suppressed macaques. Immunity. 2023;56(7):1649-63 e5. Epub 20230525. doi: 10.1016/j.immuni.2023.04.019. PubMed PMID: 37236188; PMCID: PMC10524637.
142. Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, Storb R. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068-73. doi: 10.1056/NEJM197905103001902. PubMed PMID: 34792.
143. Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED, Seattle Marrow Transplant T. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304(25):1529-33. doi: 10.1056/NEJM198106183042507. PubMed PMID: 7015133.
144. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, Ciceri F, Cornelissen J, Malladi R, Duarte RF, Giebel S, Greinix H, Holler E, Lawitschka A, Mielke S, Mohty M, Arat M, Nagler A, Passweg J, Schoemans H, Socie G, Solano C, Vrhovac R, Zeiser R, Kroger N, Basak GW. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7(2):e157-e67. doi: 10.1016/S2352-3026(19)30256-X. PubMed PMID: 32004485.
145. Yoshinaga T, Miki S, Kawauchi-Miki S, Seki T, Fujiwara T. Barrier to Resistance of Dolutegravir in Two-Drug Combinations. Antimicrob Agents Chemother. 2019;63(3). Epub 20190226. doi: 10.1128/AAC.02104-18. PubMed PMID: 30602514; PMCID: PMC6395897.
146. Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy? J Antimicrob Chemother. 2014;69(7):1742-7. Epub 20140305. doi: 10.1093/jac/dku058. PubMed PMID: 24603962.
147. Nickel K, Halfpenny NJA, Snedecor SJ, Punekar YS. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-naive patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis. 2021;21(1):222. Epub 20210226. doi: 10.1186/s12879-021-05850-0. PubMed PMID: 33637050; PMCID: PMC7908737.
148. Markowitz M, Zolopa A, Squires K, Ruane P, Coakley D, Kearney B, Zhong L, Wulfsohn M, Miller MD, Lee WA. Phase I/II study of the pharmacokinetics, safety and antiretroviral activity of tenofovir alafenamide, a new prodrug of the HIV reverse transcriptase inhibitor tenofovir, in HIV-infected adults. J Antimicrob Chemother. 2014;69(5):1362-9. Epub 20140206. doi: 10.1093/jac/dkt532. PubMed PMID: 24508897.
149. Callebaut C, Stepan G, Tian Y, Miller MD. In Vitro Virology Profile of Tenofovir Alafenamide, a Novel Oral Prodrug of Tenofovir with Improved Antiviral Activity Compared to That of Tenofovir Disoproxil Fumarate. Antimicrob Agents Chemother. 2015;59(10):5909-16. Epub 20150706. doi: 10.1128/AAC.01152-15. PubMed PMID: 26149992; PMCID: PMC4576064.
150. Diamond TL, Ngo W, Xu M, Goh SL, Rodriguez S, Lai MT, Asante-Appiah E, Grobler JA. Islatravir Has a High Barrier to Resistance and Exhibits a Differentiated Resistance Profile from Approved Nucleoside Reverse Transcriptase Inhibitors (NRTIs). Antimicrob Agents Chemother. 2022;66(6):e0013322. Epub 20220512. doi: 10.1128/aac.00133-22. PubMed PMID: 35546110; PMCID: PMC9211433.
151. Matthews RP, Ankrom W, Friedman E, Jackson Rudd D, Liu Y, Mogg R, Panebianco D, De Lepeleire I, Petkova M, Grobler JA, Stoch SA, Iwamoto M. Safety, tolerability, and pharmacokinetics of single- and multiple-dose administration of islatravir (MK-8591) in adults without HIV. Clin Transl Sci. 2021;14(5):1935-44. Epub 20210831. doi: 10.1111/cts.13048. PubMed PMID: 34463432; PMCID: PMC8504818.
152. Matthews RP, Patel M, Barrett SE, Haspeslagh L, Reynders T, Zhang S, Rottey S, Goodey A, Vargo RC, Grobler JA, Stoch SA, Iwamoto M. Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial. Nat Med. 2021;27(10):1712-7. Epub 20211004. doi: 10.1038/s41591-021-01479-3. PubMed PMID: 34608329.
153. Link JO, Rhee MS, Tse WC, Zheng J, Somoza JR, Rowe W, Begley R, Chiu A, Mulato A, Hansen D, Singer E, Tsai LK, Bam RA, Chou CH, Canales E, Brizgys G, Zhang JR, Li J, Graupe M, Morganelli P, Liu Q, Wu Q, Halcomb RL, Saito RD, Schroeder SD, Lazerwith SE, Bondy S, Jin D, Hung M, Novikov N, Liu X, Villasenor AG, Cannizzaro CE, Hu EY, Anderson RL, Appleby TC, Lu B, Mwangi J, Liclican A, Niedziela-Majka A, Papalia GA, Wong MH, Leavitt SA, Xu Y, Koditek D, Stepan GJ, Yu H, Pagratis N, Clancy S, Ahmadyar S, Cai TZ, Sellers S, Wolckenhauer SA, Ling J, Callebaut C, Margot N, Ram RR, Liu YP, Hyland R, Sinclair GI, Ruane PJ, Crofoot GE, McDonald CK, Brainard DM, Lad L, Swaminathan S, Sundquist WI, Sakowicz R, Chester AE, Lee WE, Daar ES, Yant SR, Cihlar T. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature. 2020;584(7822):614-8. Epub 20200701. doi: 10.1038/s41586-020-2443-1. PubMed PMID: 32612233; PMCID: PMC8188729.
154. Segal-Maurer S, DeJesus E, Stellbrink HJ, Castagna A, Richmond GJ, Sinclair GI, Siripassorn K, Ruane PJ, Berhe M, Wang H, Margot NA, Dvory-Sobol H, Hyland RH, Brainard DM, Rhee MS, Baeten JM, Molina JM, Investigators CS. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N Engl J Med. 2022;386(19):1793-803. doi: 10.1056/NEJMoa2115542. PubMed PMID: 35544387.
155. Balibar CJ, Klein DJ, Zamlynny B, Diamond TL, Fang Z, Cheney CA, Kristoff J, Lu M, Bukhtiyarova M, Ou Y, Xu M, Ba L, Carroll SS, El Marrouni A, Fay JF, Forster A, Goh SL, Gu M, Krosky D, Rosenbloom DIS, Sheth P, Wang D, Wu G, Zebisch M, Zhao T, Zuck P, Grobler J, Hazuda DJ, Howell BJ, Converso A. Potent targeted activator of cell kill molecules eliminate cells expressing HIV-1. Sci Transl Med. 2023;15(684):eabn2038. Epub 20230222. doi: 10.1126/scitranslmed.abn2038. PubMed PMID: 36812345.
156. Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masia M, Latiff G, Pokrovsky V, Bredeek F, Smith G, Cahn P, Kim YS, Ford SL, Talarico CL, Patel P, Chounta V, Crauwels H, Parys W, Vanveggel S, Mrus J, Huang J, Harrington CM, Hudson KJ, Margolis DA, Smith KY, Williams PE, Spreen WR. Long-Acting Cabotegravir and Rilpivirine for Maintenance of HIV-1 Suppression. N Engl J Med. 2020;382(12):1112-23. Epub 20200304. doi: 10.1056/NEJMoa1904398. PubMed PMID: 32130809.
157. Orkin C, Arasteh K, Gorgolas Hernandez-Mora M, Pokrovsky V, Overton ET, Girard PM, Oka S, Walmsley S, Bettacchi C, Brinson C, Philibert P, Lombaard J, St Clair M, Crauwels H, Ford SL, Patel P, Chounta V, D’Amico R, Vanveggel S, Dorey D, Cutrell A, Griffith S, Margolis DA, Williams PE, Parys W, Smith KY, Spreen WR. Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection. N Engl J Med. 2020;382(12):1124-35. Epub 20200304. doi: 10.1056/NEJMoa1909512. PubMed PMID: 32130806.
158. Jaeger H, Overton ET, Richmond G, Rizzardini G, Andrade-Villanueva JF, Mngqibisa R, Hermida AO, Thalme A, Belonosova E, Ajana F, Benn PD, Wang Y, Hudson KJ, Espanol CM, Ford SL, Crauwels H, Margolis DA, Talarico CL, Smith KY, van Eygen V, Van Solingen-Ristea R, Vanveggel S, Spreen WR. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV. 2021;8(11):e679-e89. Epub 20211011. doi: 10.1016/S2352-3018(21)00185-5. PubMed PMID: 34648734.
159. Gupta SK, Berhe M, Crofoot G, Benson P, Ramgopal M, Sims J, McDonald C, Ruane P, Sanchez WE, Scribner A, Liu SY, VanderVeen LA, Dvory-Sobol H, Rhee MS, Baeten JM, Koenig E. Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: a randomised, open-label, active-controlled, phase 2 trial. Lancet HIV. 2023;10(1):e15-e23. doi: 10.1016/S2352-3018(22)00291-0. PubMed PMID: 36566079.
160. Ogbuagu O, Segal-Maurer S, Ratanasuwan W, Avihingsanon A, Brinson C, Workowski K, Antinori A, Yazdanpanah Y, Trottier B, Wang H, Margot N, Dvory-Sobol H, Rhee MS, Baeten JM, Molina JM, investigators G-U-. Efficacy and safety of the novel capsid inhibitor lenacapavir to treat multidrug-resistant HIV: week 52 results of a phase 2/3 trial. Lancet HIV. 2023;10(8):e497-e505. Epub 20230711. doi: 10.1016/S2352-3018(23)00113-3. PubMed PMID: 37451297.
161. Rothemejer FH, Lauritsen NP, Juhl AK, Schleimann MH, Konig S, Sogaard OS, Bak RO, Tolstrup M. Development of HIV-Resistant CAR T Cells by CRISPR/Cas-Mediated CAR Integration into the CCR5 Locus. Viruses. 2023;15(1). Epub 20230110. doi: 10.3390/v15010202. PubMed PMID: 36680242; PMCID: PMC9862650.
162. High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR, Jr., Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P, HIV OARWGo, Aging. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60 Suppl 1(Suppl 1):S1-18. doi: 10.1097/QAI.0b013e31825a3668. PubMed PMID: 22688010; PMCID: PMC3413877.
163. Masters MC, Landay AL, Robbins PD, Tchkonia T, Kirkland JL, Kuchel GA, Niedernhofer LJ, Palella FJ. Chronic HIV Infection and Aging: Application of a Geroscience-Guided Approach. J Acquir Immune Defic Syndr. 2022;89(Suppl 1):S34-S46. doi: 10.1097/QAI.0000000000002858. PubMed PMID: 35015744; PMCID: PMC8751288.
164. Grifoni A, Alonzi T, Alter G, Noonan DM, Landay AL, Albini A, Goletti D. Impact of aging on immunity in the context of COVID-19, HIV, and tuberculosis. Front Immunol. 2023;14:1146704. Epub 20230524. doi: 10.3389/fimmu.2023.1146704. PubMed PMID: 37292210; PMCID: PMC10246744.
165. Dalzini A, Ballin G, Dominguez-Rodriguez S, Rojo P, Petrara MR, Foster C, Cotugno N, Ruggiero A, Nastouli E, Klein N, Rinaldi S, Pahwa S, Rossi P, Giaquinto C, Palma P, De Rossi A, Consortium E. Size of HIV-1 reservoir is associated with telomere shortening and immunosenescence in early-treated European children with perinatally acquired HIV-1. J Int AIDS Soc. 2021;24(11):e25847. doi: 10.1002/jia2.25847. PubMed PMID: 34797948; PMCID: PMC8604380.
166. Alcaide ML, Parmigiani A, Pallikkuth S, Roach M, Freguja R, Della Negra M, Bolivar H, Fischl MA, Pahwa S. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8(5):e63804. Epub 20130528. doi: 10.1371/journal.pone.0063804. PubMed PMID: 23724003; PMCID: PMC3665816.
167. Alvarez S, Branas F, Sanchez-Conde M, Moreno S, Lopez-Bernaldo de Quiros JC, Munoz-Fernandez MA. Frailty, markers of immune activation and oxidative stress in HIV infected elderly. PLoS One. 2020;15(3):e0230339. Epub 20200318. doi: 10.1371/journal.pone.0230339. PubMed PMID: 32187205; PMCID: PMC7080240.
168. Aung HL, Bloch M, Vincent T, Mao L, Brew BJ, Cysique LA. Low incidence of advanced neurological burden but high incidence of age-related conditions that are dementia risk factors in aging people living with HIV: a data-linkage 10-year follow-up study. J Neurovirol. 2023;29(2):141-55. Epub 20221212. doi: 10.1007/s13365-022-01104-0. PubMed PMID: 36508059; PMCID: PMC10185650.
169. Soares LS, Espindola MS, Zambuzi FA, Galvao-Lima LJ, Cacemiro MC, Soares MR, Santana BA, Calado RT, Bollela VR, Frantz FG. Immunosenescence in chronic HIV infected patients impairs essential functions of their natural killer cells. Int Immunopharmacol. 2020;84:106568. Epub 20200511. doi: 10.1016/j.intimp.2020.106568. PubMed PMID: 32408187.
170. Shin MS, Park HJ, Salahuddin S, Montgomery RR, Emu B, Shaw AC, Kang I. Alterations in high-dimensional T-cell profile and gene signature of immune aging in HIV-infected older adults without viremia. Aging Cell. 2022;21(10):e13702. Epub 20220829. doi: 10.1111/acel.13702. PubMed PMID: 36036630; PMCID: PMC9577958.
171. Hove-Skovsgaard M, Zhao Y, Tingstedt JL, Hartling HJ, Thudium RF, Benfield T, Afzal S, Nordestgaard B, Ullum H, Gerstoft J, Mocroft A, Nielsen SD. Impact of Age and HIV Status on Immune Activation, Senescence and Apoptosis. Front Immunol. 2020;11:583569. Epub 20200930. doi: 10.3389/fimmu.2020.583569. PubMed PMID: 33117394; PMCID: PMC7561401.
172. Szaniawski MA, Spivak AM. Senotherapeutics for HIV and aging. Curr Opin HIV AIDS. 2020;15(2):83-93. doi: 10.1097/COH.0000000000000609. PubMed PMID: 31833962; PMCID: PMC7325840.
173. Sanchez-Diaz L, Espinosa-Sanchez A, Blanco JR, Carnero A. Senotherapeutics in Cancer and HIV. Cells. 2022;11(7). Epub 20220404. doi: 10.3390/cells11071222. PubMed PMID: 35406785; PMCID: PMC8997781.
174. Morales DR, Moreno-Martos D, Matin N, McGettigan P. Health conditions in adults with HIV compared with the general population: A population-based cross-sectional analysis. EClinicalMedicine. 2022;47:101392. Epub 20220421. doi: 10.1016/j.eclinm.2022.101392. PubMed PMID: 35497059; PMCID: PMC9046106.
175. Abdel-Hakeem MS, Manne S, Beltra JC, Stelekati E, Chen Z, Nzingha K, Ali MA, Johnson JL, Giles JR, Mathew D, Greenplate AR, Vahedi G, Wherry EJ. Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation. Nat Immunol. 2021;22(8):1008-19. Epub 20210726. doi: 10.1038/s41590-021-00975-5. PubMed PMID: 34312545; PMCID: PMC8323971.
176. Yates KB, Tonnerre P, Martin GE, Gerdemann U, Al Abosy R, Comstock DE, Weiss SA, Wolski D, Tully DC, Chung RT, Allen TM, Kim AY, Fidler S, Fox J, Frater J, Lauer GM, Haining WN, Sen DR. Epigenetic scars of CD8(+) T cell exhaustion persist after cure of chronic infection in humans. Nat Immunol. 2021;22(8):1020-9. Epub 20210726. doi: 10.1038/s41590-021-00979-1. PubMed PMID: 34312547; PMCID: PMC8600539.
177. Patrinely JR, Jr., Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, Yeoh HL, Palmeri M, Ye F, Fan R, Davis EJ, Rapisuwon S, Long GV, Haydon A, Osman I, Mehnert JM, Carlino MS, Sullivan RJ, Menzies AM, Johnson DB. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021;7(5):744-8. doi: 10.1001/jamaoncol.2021.0051. PubMed PMID: 33764387; PMCID: PMC7995124.
178. Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254-67. Epub 20220126. doi: 10.1038/s41571-022-00600-w. PubMed PMID: 35082367; PMCID: PMC8790946.
179. Baker KS, Leisenring WM, Goodman PJ, Ermoian RP, Flowers ME, Schoch G, Storb R, Sandmaier BM, Deeg HJ. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood. 2019;133(26):2790-9. Epub 20190416. doi: 10.1182/blood.2018874115. PubMed PMID: 30992266; PMCID: PMC6598379.
180. Hussaini SMQ, Gupta A, Dusetzina SB. Financial Toxicity of Cancer Treatment. JAMA Oncol. 2022;8(5):788. doi: 10.1001/jamaoncol.2021.7987. PubMed PMID: 35266966.
181. Workman P, Draetta GF, Schellens JHM, Bernards R. How Much Longer Will We Put Up With $100,000 Cancer Drugs? Cell. 2017;168(4):579-83. doi: 10.1016/j.cell.2017.01.034. PubMed PMID: 28187281.
182. Vokinger KN, Hwang TJ, Daniore P, Lee CC, Tibau A, Grischott T, Rosemann TJ, Kesselheim AS. Analysis of Launch and Postapproval Cancer Drug Pricing, Clinical Benefit, and Policy Implications in the US and Europe. JAMA Oncol. 2021;7(9):e212026. Epub 20210916. doi: 10.1001/jamaoncol.2021.2026. PubMed PMID: 34196656; PMCID: PMC8251654.
183. Schaft N, Dorrie J, Schuler G, Schuler-Thurner B, Sallam H, Klein S, Eisenberg G, Frankenburg S, Lotem M, Khatib A. The future of affordable cancer immunotherapy. Front Immunol. 2023;14:1248867. Epub 20230906. doi: 10.3389/fimmu.2023.1248867. PubMed PMID: 37736099; PMCID: PMC10509759.
184. Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, Foell J, de la Fuente J, Grupp S, Handgretinger R, Ho TW, Kattamis A, Kernytsky A, Lekstrom-Himes J, Li AM, Locatelli F, Mapara MY, de Montalembert M, Rondelli D, Sharma A, Sheth S, Soni S, Steinberg MH, Wall D, Yen A, Corbacioglu S. CRISPR-Cas9 Gene Editing for Sickle Cell Disease and beta-Thalassemia. N Engl J Med. 2021;384(3):252-60. Epub 20201205. doi: 10.1056/NEJMoa2031054. PubMed PMID: 33283989.
185. Kanter J, Walters MC, Krishnamurti L, Mapara MY, Kwiatkowski JL, Rifkin-Zenenberg S, Aygun B, Kasow KA, Pierciey FJ, Jr., Bonner M, Miller A, Zhang X, Lynch J, Kim D, Ribeil JA, Asmal M, Goyal S, Thompson AA, Tisdale JF. Biologic and Clinical Efficacy of LentiGlobin for Sickle Cell Disease. N Engl J Med. 2022;386(7):617-28. Epub 20211212. doi: 10.1056/NEJMoa2117175. PubMed PMID: 34898139.
186. Martinez-Navio JM, Fuchs SP, Pantry SN, Lauer WA, Duggan NN, Keele BF, Rakasz EG, Gao G, Lifson JD, Desrosiers RC. Adeno-Associated Virus Delivery of Anti-HIV Monoclonal Antibodies Can Drive Long-Term Virologic Suppression. Immunity. 2019;50(3):567-75 e5. Epub 20190305. doi: 10.1016/j.immuni.2019.02.005. PubMed PMID: 30850342; PMCID: PMC6457122.
187. Casazza JP, Cale EM, Narpala S, Yamshchikov GV, Coates EE, Hendel CS, Novik L, Holman LA, Widge AT, Apte P, Gordon I, Gaudinski MR, Conan-Cibotti M, Lin BC, Nason MC, Trofymenko O, Telscher S, Plummer SH, Wycuff D, Adams WC, Pandey JP, McDermott A, Roederer M, Sukienik AN, O’Dell S, Gall JG, Flach B, Terry TL, Choe M, Shi W, Chen X, Kaltovich F, Saunders KO, Stein JA, Doria-Rose NA, Schwartz RM, Balazs AB, Baltimore D, Nabel GJ, Koup RA, Graham BS, Ledgerwood JE, Mascola JR, Team VRCS. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat Med. 2022;28(5):1022-30. Epub 20220411. doi: 10.1038/s41591-022-01762-x. PubMed PMID: 35411076; PMCID: PMC9876739.
188. Priddy FH, Lewis DJM, Gelderblom HC, Hassanin H, Streatfield C, LaBranche C, Hare J, Cox JH, Dally L, Bendel D, Montefiori D, Sayeed E, Ackland J, Gilmour J, Schnepp BC, Wright JF, Johnson P. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV. 2019;6(4):e230-e9. Epub 20190315. doi: 10.1016/S2352-3018(19)30003-7. PubMed PMID: 30885692; PMCID: PMC6443625.
189. Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D, Kariko K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010;38(17):5884-92. Epub 20100510. doi: 10.1093/nar/gkq347. PubMed PMID: 20457754; PMCID: PMC2943593.
190. Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165-75. doi: 10.1016/j.immuni.2005.06.008. PubMed PMID: 16111635.
191. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-16. Epub 20201230. doi: 10.1056/NEJMoa2035389. PubMed PMID: 33378609; PMCID: PMC7787219.
192. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-15. Epub 20201210. doi: 10.1056/NEJMoa2034577. PubMed PMID: 33301246; PMCID: PMC7745181.
193. Wei T, Cheng Q, Min YL, Olson EN, Siegwart DJ. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat Commun. 2020;11(1):3232. Epub 20200626. doi: 10.1038/s41467-020-17029-3. PubMed PMID: 32591530; PMCID: PMC7320157.
194. Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15(4):313-20. Epub 20200406. doi: 10.1038/s41565-020-0669-6. PubMed PMID: 32251383; PMCID: PMC7735425.
195. McCune JM, Turner EH, Jiang A, Doehle BP. Bringing Gene Therapies for HIV Disease to Resource-Limited Parts of the World. Hum Gene Ther. 2021;32(1-2):21-30. Epub 20201030. doi: 10.1089/hum.2020.252. PubMed PMID: 32998595; PMCID: PMC10112459.
196. Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2022;2022(1):569-78. doi: 10.1182/hematology.2022000388. PubMed PMID: 36485127; PMCID: PMC9821304.
197. Kasiewicz LN, Biswas S, Beach A, Ren H, Dutta C, Mazzola AM, Rohde E, Chadwick A, Cheng C, Garcia SP, Iyer S, Matsumoto Y, Khera AV, Musunuru K, Kathiresan S, Malyala P, Rajeev KG, Bellinger AM. GalNAc-Lipid nanoparticles enable non-LDLR dependent hepatic delivery of a CRISPR base editing therapy. Nat Commun. 2023;14(1):2776. Epub 20230515. doi: 10.1038/s41467-023-37465-1. PubMed PMID: 37188660; PMCID: PMC10185539.
198. Gaiha GD, Rossin EJ, Urbach J, Landeros C, Collins DR, Nwonu C, Muzhingi I, Anahtar MN, Waring OM, Piechocka-Trocha A, Waring M, Worrall DP, Ghebremichael MS, Newman RM, Power KA, Allen TM, Chodosh J, Walker BD. Structural topology defines protective CD8(+) T cell epitopes in the HIV proteome. Science. 2019;364(6439):480-4. doi: 10.1126/science.aav5095. PubMed PMID: 31048489; PMCID: PMC6855781.
199. Beacroft L, Hallett TB. The potential impact of a “curative intervention” for HIV: a modelling study. Glob Health Res Policy. 2019;4:2. Epub 20190612. doi: 10.1186/s41256-019-0107-1. PubMed PMID: 31223659; PMCID: PMC6567561.
200. Dybul M, Attoye T, Baptiste S, Cherutich P, Dabis F, Deeks SG, Dieffenbach C, Doehle B, Goodenow MM, Jiang A, Kemps D, Lewin SR, Lumpkin MM, Mathae L, McCune JM, Ndung’u T, Nsubuga M, Peay HL, Pottage J, Warren M, Sikazwe I, Sunnylands Working G. The case for an HIV cure and how to get there. Lancet HIV. 2021;8(1):e51-e8. Epub 20201130. doi: 10.1016/S2352-3018(20)30232-0. PubMed PMID: 33271124; PMCID: PMC7773626.
201. McCune JM, Stevenson SC, Doehle BP, Trenor CC, 3rd, Turner EH, Spector JM. Collaborative science to advance gene therapies in resource-limited parts of the world. Mol Ther. 2021;29(11):3101-2. Epub 20210830. doi: 10.1016/j.ymthe.2021.05.024. PubMed PMID: 34464598; PMCID: PMC8571166.
Submitted January 3, 2024 | Accepted February 14, 2024 | Published March 1, 2024
Copyright © 2024 The Authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.