Sneha Datwani1, Rebecca Kalikawe1, Francis Mwimanzi1, Sarah Speckmaier2, Richard Liang2, Yurou Sang1, Rachel Waterworth1, Fatima Yaseen3, Hope R. Lapointe2, Evan Barad1,2, Mari L. DeMarco4,5, Daniel T. Holmes4,5, Janet Simons4,5, Julio S.G. Montaner3,6, Marc G. Romney4,5, Zabrina L. Brumme1,2*, Mark A. Brockman1,2,3,*
1 Faculty of Health Sciences, Simon Fraser University, Burnaby, Canada
2 British Columbia Centre for Excellence in HIV/AIDS, Vancouver, Canada
3 Department of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, Canada
4 Division of Medical Microbiology and Virology, St. Paul’s Hospital, Vancouver, Canada
5 Department of Pathology and Laboratory Medicine, University of British Columbia, Vancouver, Canada
6 Department of Medicine, University of British Columbia, Vancouver, Canada
*These authors contributed equally
Mark A. Brockman
mark_brockman@sfu.ca
Zabrina L. Brumme
zbrumme@sfu.ca
Datwani S, Kalikawe R, Mwimanzi K, Speckmaier S, Liang R, Sang Y, Waterworth R, Yaseen F, Lapointe HR, Barad E, DeMarco ML, Holmes DT, Simons J, Montaner JSG, Romney MG, Brumme ZL, Brockman MA. Dynamics of T-cell Responses Following COVID-19 mRNA Vaccination and Breakthrough Infection in Older Adults.Pathogens and Immunity. 2023;8(1):117–135. doi: 10.20411/pai.v8i1.613
10.20411/pai.v8i1.613
Introduction: While older adults generally mount weaker antibody responses to a primary COVID-19 vaccine series, T-cell responses remain less well characterized in this population. We compared SARS-CoV-2 spike-specific T-cell responses after 2- and 3-dose COVID-19 mRNA vaccination and subsequent breakthrough infection in older and younger adults.
Methods: We quantified CD4+ and CD8+ T-cells reactive to overlapping peptides spanning the ancestral SARS-CoV-2 spike protein in 40 older adults (median age 79) and 50 younger health care workers (median age 39), all COVID-19 naive, using an activation-induced marker assay. T-cell responses were further assessed in 24 participants, including 8 older adults, who subsequently experienced their first SARS-CoV-2 breakthrough infection.
Results: A third COVID-19 mRNA vaccine dose significantly boosted spike-specific CD4+ and CD8+ T-cell frequencies to above 2-dose levels in older and younger adults. T-cell frequencies did not significantly differ between older and younger adults after either dose. Multivariable analyses adjusting for sociodemographic, health, and vaccine-related variables confirmed that older age was not associated with impaired cellular responses. Instead, the strongest predictors of CD4+ and CD8+ T-cell frequencies post-third-dose were their corresponding post-second-dose frequencies. Breakthrough infection significantly increased both CD4+ and CD8+ T-cell frequencies, to comparable levels in older and younger adults. Exploratory analyses revealed an association between HLA-A*02:03 and higher post-vaccination CD8+ T-cell frequencies, which may be attributable to numerous strong-binding HLA-A*02:03-specific CD8+ T-cell epitopes in the spike protein.
Conclusion: Older adults mount robust T-cell responses to 2- and 3-dose COVID-19 mRNA vaccination, which are further boosted following breakthrough infection.
COVID-19; mRNA vaccines; cellular immune response; older adults; activation-induced marker assay
In many jurisdictions, older adults were prioritized to receive COVID-19 vaccines and boosters due to their increased risk of severe outcomes following SARS-CoV-2 infection [1–3]. While vaccination has been highly effective at preventing severe disease in this group [4], vaccine responses in older adults can nevertheless be blunted by age-related immune impairments, or elevated frequencies of chronic health conditions that can dampen adaptive immune responses [5–9]. Indeed, after COVID-19 vaccines were rolled out globally, observational studies revealed that older adults generally mounted weaker binding and neutralizing antibody to the primary vaccine series [10–15], leading to widespread recommendations that this group receive third vaccine doses and regular booster vaccinations [16, 17]. Comparably fewer studies however have investigated cellular immune responses to COVID-19 vaccination−namely, CD4+ helper T cells that play a central role in the generation of antigen-specific B cells and antibody responses, and CD8+ cytotoxic T cells that recognize and eliminate virus-infected cells [18] − in older compared to younger adults. While mRNA vaccines can induce strong T-cell responses [19, 20], evidence suggests that the frequency of spike-specific CD4+ T cells following COVID-19 vaccination may be lower in older adults [11, 13, 21]. An improved understanding of age-associated differences in T-cell responses to COVID-19 mRNA vaccines will help to inform future efforts to enhance protective immunity in older adults.
Here, we investigated the dynamics of spike-specific CD4+ and CD8+ T-cell responses elicited after 2- and 3-dose COVID-19 mRNA vaccination in a cohort of 40 older adults and 50 younger healthcare workers who remained naive to SARS-CoV-2 during this time. We additionally investigated spike-specific CD4+ and CD8+ T-cell responses in a subset of 24 individuals, including 8 older adults, who subsequently experienced their first SARS-CoV-2 breakthrough infection between 1 and 6 months after receiving 3 vaccine doses. Finally, we explored associations between HLA class I allele carriage and the magnitude of spike-specific CD8+ T-cell frequencies after 2- and 3- dose COVID-19 mRNA vaccination.
Our cohort, based in British Columbia Canada, has been described previously [22]. Here, we studied a randomly selected subset of 50 healthcare workers (HCW) and 40 older adults (OA, age >65 years) who remained COVID-19 naive until at least 1 month after their third COVID-19 mRNA vaccine dose (Table 1).
Written informed consent was obtained from all participants or their authorized decision makers. This study was approved by the University of British Columbia/Providence Health Care and Simon Fraser University Research Ethics Boards.
We had previously quantified IgG-binding antibodies in serum against the ancestral SARS-CoV-2 spike receptor binding domain (RBD) using the V-plex SARS-CoV-2 (IgG) ELISA kit (Panel 22; Meso Scale Diagnostics)[14, 15, 22]. Serum was diluted 1:10000 and reported in World Health Organization (WHO) International Standard Binding Antibody Units (BAU)/mL using the manufacturer-supplied conversions. SARS-CoV-2 infections were detected by the development of serum antibodies against Nucleocapsid (N) using the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics), combined with diagnostic (PCR- and/or rapid-antigen-test-based) information where available.
Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and diluted in TexMACS media (Miltenyi Biotec, Cat#130-097-196). PBMCs were stimulated at 1 x 106 cells per well for 24 hours with peptide pools spanning the SARS-CoV-2 ancestral spike protein (15-mers, overlapping by 11 amino acids) (Miltenyi Biotec, Cat#130-127-953) in duplicate in a 96-well U-bottom plate. PBMC were incubated with DMSO only (no peptide) as a negative control and 2 µg/mL Cytostim reagent (Miltenyi Biotec, Cat#130-092-172) as a positive control. Following stimulation, cells were labeled with CD8-APC/Cyanine7 (Biolegend, Cat#301016), CD4-FITC (Biolegend, Cat#300538), CD137-APC (Biolegend, Cat#309810), CD69-PE (BD, Cat#555531), OX40-PE-Cy7 (Biolegend, Cat#350012), CD3-PerCP/Cyanine5.5 (Biolegend, Cat#317336) CD14-V500 (BD, Cat#561391), CD19-V500 (BD, Cat#561121) and 7-AAD Viability Staining Solution (Biolegend, Cat#420404). Data were acquired on a Beckman Coulter Cytoflex flow cytometer, with a minimum of 10,000 CD3+ T cells assayed per participant. After identifying CD3+CD4+ and CD3+CD8+ T-cell subsets, the percentage of stimulated cells was determined based on upregulation of activation markers, using CD137 and OX40 for CD4+ T cells and CD137 and CD69 for CD8+ T cells (see gating strategy in Supplementary Figure 1). Data were analyzed in FlowJo version 10.8.1.
Genomic DNA was isolated from 50 μL whole blood using the NucliSENS EasyMag system (BioMerieux). HLA class I genotyping was performed by locus-specific Polymerase Chain Reaction (PCR) amplification of the region spanning exons 2 and 3 of the HLA-A, -B, and -C loci, as reported previously [23]. Amplicons were bi-directionally sequenced on an ABI 3730xl automated Sanger DNA sequencer using BigDye (v3.1) chemistry (Applied Biosystems). Chromatograms were analyzed using the semiautomatic base-calling software RECall [24], with the resulting bulk sequences interpreted to subtype-level resolution using in-house software.
Peptides derived from ancestral spike protein (GenBank: NC_045512.2) that are likely to bind to HLA-A*02:01 and/or A*02:03 were predicted using NetMHCpan-4.1 (https://services.healthtech.dtu.dk/services/NetMHCpan-4.1; [25]). Potential 8-, 9-, and 10- amino acid epitopes were determined for both alleles using default thresholds for strong binders (% Rank <0.5) and weak binders (% Rank 0.5-2).
Continuous variables were compared using the Mann-Whitney U-test (for unpaired data) or Wilcoxon test (for paired measures). Relationships between continuous variables were assessed using Spearman’s correlation. Zero-inflated beta regressions were used to investigate the relationship between age and vaccine-induced T-cell responses using a confounder model that adjusted for variables that could influence these responses, or that differed in prevalence between groups. These regressions model the response variable as a beta-distributed random variable whose mean is given by a linear combination of the predictor variables (after a logit transformation). Beta distributions are bounded below and above by 0 and 100%, making this a standard choice of regression for frequency data. A beta distribution, however, does not admit values of 0 or 100. As our data included some non-responders (ie, 0 values) we used a zero-inflated beta distribution, which allows for zeros in the data. For analyses performed after 2-dose vaccination, included variables were age (per year), sex at birth (female as reference), ethnicity (non-white as reference), major chronic conditions (defined as cancer or blood disorders; no conditions as reference), other chronic conditions (no conditions as reference), mRNA-1273-containing initial vaccine regimen (dual BNT162b2 vaccination as reference), and the interval between first and second doses (per day). Analyses performed after 3-dose vaccination also included the third COVID-19 mRNA dose brand (BNT162b2 as reference), the interval between second and third doses (per day), and the % spike-specific T-cells after 2 doses (by percent increase). All tests were 2-tailed, with P<0.05 considered statistically significant. For the analyses of the relationships between HLA class I allele carriage, multiple comparisons were addressed using a Q-value (false discovery rate) approach [26], with associations P<0.05 and Q<0.2 considered statistically significant. Analyses were conducted using Prism v9.2.0 (GraphPad) and in R.
Characteristics of the 40 older adults (OA) and 50 younger health care workers (HCW) are shown in Table 1. Based on repeated negative results for serologic testing of SARS-CoV-2 anti-N antibodies, all participants remained COVID-19 naive until at least 1-month after their third vaccine dose. OA and HCW were a median of 79 and 39 years old, respectively (overall range 24-93 years old), and predominantly female. OA were predominantly of white ethnicity (73%, compared to 46% of HCW) and had more chronic health conditions (median of 1, interquartile range [IQR] 0-2 in OA versus 0 [IQR 0-1] in HCW). Most participants (80% of OA and 96% of HCW) initially received 2 doses of BNT162b2; the remainder received 2 doses of mRNA-1273 or a heterologous mRNA vaccine regimen. Second doses were administered a median of ~3 months after the first. Due to the limited availability of mRNA vaccines in late 2020 and early 2021, British Columbia employed a delayed second-dose strategy that differed from the manufacturers’ recommended interval of 21-28 days, which was commonly used in the United States. Recent studies have indicated that delaying second doses did not have a detrimental impact on vaccine efficacy, and in some cases may enhance protection against infection or hospitalization [27] and reduce the risk of myocarditis [28]. Indeed, guidance from the World Health Organization now recommends a 2-month interval between first and second doses [29]. Third vaccine doses were predominantly mRNA-1273 (62% of OA and 50% of HCW), which were administered in an age-dependent manner according to local guidelines: specifically, OA were eligible for 100 mcg whereas HCW were eligible for 50 mcg (while all BNT162b2 doses were 30 mcg). Third doses were administered a median of 6 months after the second. A total of 20% of OA and 56% of HCW experienced their first SARS-CoV-2 infection between 1 and 6 months post-third dose, where these were likely Omicron BA.1 or BA.2 infections based on local molecular epidemiology trends [30]. The post-infection follow-up visit occurred 53 (IQR 40-119) days later for OA and 63 (IQR 31-104) days later for HCW.
Table 1: Participant Characteristics
|
Characteristic |
OA (n=40) |
HCW (n=50) |
|
Sociodemographic and health variablesa |
||
|
Age in years, median [IQR] |
79 [73-83] |
39 [32-50] |
|
Female sex at birth, n (%) |
27 (68%) |
37 (74%) |
|
Ethnicity, n (%) White, n (%) |
|
|
|
Asian, n (%) |
10 (25%) |
22 (44%) |
|
Other, n (%) |
1 (2%) |
5 (10%) |
|
Number of chronic health conditions, median [IQR]b |
1 [0-2] |
0 [0-1] |
|
Hypertension, n (%) |
17 (43%) |
5 (10%) |
|
Diabetes, n (%) |
10 (25%) |
0 (0%) |
|
Asthma, n (%) |
3 (7.5%) |
3 (6%) |
|
Obesity, n (%) |
5 (12.5%) |
7 (14%) |
|
Chronic lung disease, n (%) |
5 (12.5%) |
0 (0%) |
|
Chronic liver disease, n (%) |
0 (0%) |
0 (0%) |
|
Chronic kidney disease, n (%) |
5 (12.5%) |
0 (0%) |
|
Chronic heart disease, n (%) |
8 (20%) |
0 (0%) |
|
Chronic blood disease, n (%) |
1 (2.5%) |
1 (2%) |
|
Cancer, n (%) |
5 (12.5%) |
0 (0%) |
|
Immunosuppression, n (%) |
0 (0%) |
0 (0%) |
|
Vaccine details |
||
|
Initial regimen |
||
|
BNT162b2 - BNT162b2, n (%) |
32 (80%) |
48 (96%) |
|
mRNA-1273 - mRNA-1273, n (%) |
7 (18%) |
1 (2%) |
|
heterologous mRNA, n (%) |
1 (2%) |
1 (2%) |
|
Third dose |
||
|
BNT162b2, n (%) |
15 (38%) |
25 (50%) |
|
mRNA-1273, n (%) |
25 (62%) |
25 (50%) |
|
Days between first and second doses, median [IQR] |
78 [45-86] |
97 [91-101] |
|
Days between second and third doses, median [IQR] |
169 [160-231] |
210 [199-231] |
|
Post-3rd vaccine dose SARS-CoV-2 infections, n (%)c |
8 (20%) |
28 (56%) |
|
Number of breakthrough infections assessed for T-cell responses |
8 of 8 |
16 of 28 |
|
Days between breakthrough infection and T-cell assessment, median [IQR]d |
53 [40-119] |
63 [31-104] |
aSociodemographic, health, and vaccine data were collected by self-report and confirmed through medical records where available.
bChronic conditions were defined as hypertension, diabetes, asthma, obesity, chronic diseases of lung, liver, kidney, heart or blood, cancer, and immunosuppression due to chronic conditions or medication.
cAll SARS-CoV-2 infections occurred between 1 and 6 months after the third vaccine dose.
dCalculated for the participants whose T-cell responses were assessed post-breakthrough.
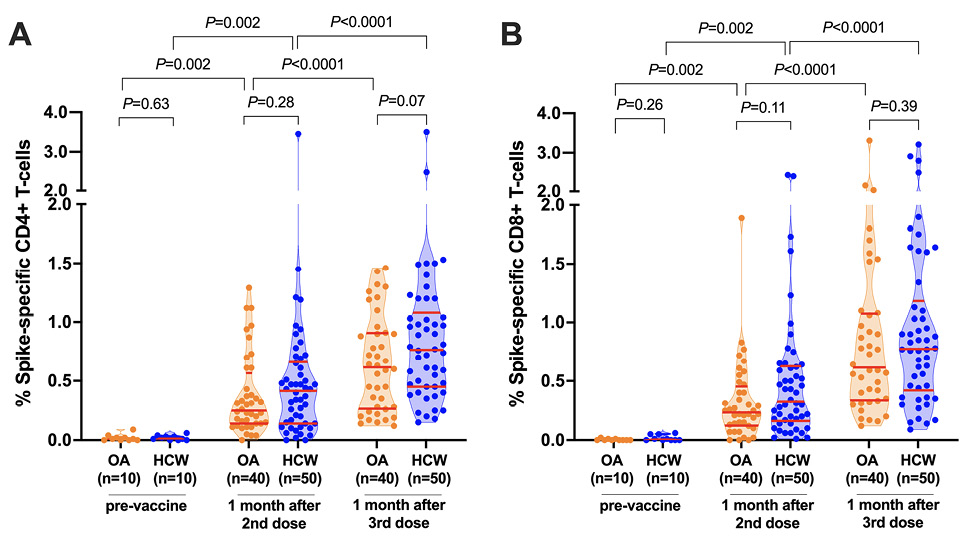
Prior to vaccination, the percentage of spike-specific CD4+ and CD8+ T cells, measured in a subset of 10 OA and 10 HCW, was negligible (Figure 1A, 1B). Following 2 vaccine doses, the percentage of spike-specific CD4+ T cells increased significantly from pre-vaccine levels (paired measures P=0.002 for both OA and HCW), reaching a median 0.25% (IQR 0.14-0.57%) in OA and a median 0.42% (IQR 0.14-0.66%) in HCW, a difference that was not statistically significant between groups (P=0.28; Figure 1A). A third vaccine dose further boosted the percentage of spike-specific CD4+ T-cells (paired measures P<0.0001 for both OA and HCW), reaching a median 0.62% (IQR 0.27-0.91) in OA compared to a median 0.77% (IQR 0.45-1.08) in HCW, a difference that was not statistically significant between groups (P=0.07) (Figure 1A).
CD8+ T-cell responses also increased significantly from pre-vaccine levels following 2 vaccine doses (paired measures P=0.002 in both groups), reaching a median 0.24% (IQR 0.13-0.45%) in OA and a median 0.33% (IQR 0.16-0.63%) in HCW, a difference that was not statistically significant between groups (P=0.11; Figure 1B). A third vaccine dose further boosted the percentage of spike-specific CD8+ T-cells (paired measures P<0.0001 for both groups) to a median 0.62% (IQR 0.34-1.08%) in OA and a median 0.78% (IQR 0.42-1.18%) in HCW, a difference that was not statistically significant between groups (P=0.39; Figure 1B).
We observed no correlation between age and the percentage of spike-specific CD4+ or CD8+ T-cells after either the second or third vaccine dose, when age was assessed as a continuous variable (Spearman’s ρ ranged from = -0.14 to -0.03; all P>0.20; not shown). Moreover, we confirmed that age remained not significantly associated with vaccine-induced T-cell responses after adjusting for relevant sociodemographic, health, and vaccine-related variables (Supplementary Tables 1 and 2). Rather, the strongest predictor of the % of spike-specific CD4+ and CD8+ T-cells following 3 vaccine doses was the corresponding % of spike-specific T-cells following 2 doses (Supplementary Table 2). For example, the zero-inflated beta regression estimates for the impact of each 1% increment in post-second dose T-cell frequencies on post-third dose frequencies were 0.71 for CD4+ T-cells (P=2×10-16) and 0.78 for CD8+ T-cells (P=6×10-14) (Supplementary Table 2).
To interpret these estimates, which do not translate the effects of the predictors linearly to the predicted mean: if the value of the linear predictor was x for a given set of values of the predictors, and the % of spike-specific CD4+ T-cells after 2 vaccine doses was increased by 1%, the resulting value of the linear predictor would now be x + 0.71, which translates to a non-linear increase in the regressed mean from 100 * logit(x) to 100 * logit(x + 0.71), where the factor of 100 converts the proportions to percentages. Enhanced CD4+ T-cell frequencies after 3 vaccine doses were also associated with male sex, having received mRNA-1273 as a third vaccine dose, and−somewhat surprisingly– the presence of chronic health conditions (omitting cancer or blood disorders), although these correlates were all weaker than the post-second-dose T-cell responses (estimates 0.23-0.29; P=0.007 to 0.03; Supplementary Table 2). We hypothesize that the association between health conditions and better T-cell responses is because individuals with such conditions benefited particularly from a third dose, after adjusting for post-second-dose responses. The association with mRNA-1273 might be due in part to the higher (100 mcg) dose that was administered to OA as the standard of care in British Columbia. White ethnicity was also weakly associated with lower CD4+ T-cell frequencies after 2 vaccine doses (estimate -0.38; P=0.03; Supplementary Table 1).

Figure 1. SARS-CoV-2 spike-specific T-cell frequencies before and after COVID-19 mRNA vaccination. Panel A: Spike-specific CD4+ T-cell frequencies before and after 2- and 3-dose COVID-19 vaccination. Older Adults (OA) are in orange; younger Health Care Workers (HCW) are in blue. All participants are COVID-19 naive. Red bars indicate median and IQR. The Mann-Whitney U-test was used for between-group comparisons and the Wilcoxon matched pairs test was used for longitudinal paired comparisons. P-values are not corrected for multiple comparisons. Panel B: Same as panel A, but for spike-specific CD8+ T-cell frequencies.
The percentage of spike-specific CD4+ and CD8+ T cells correlated strongly with one another after the second vaccine dose (Spearman’s ρ=0.60; P<0.0001) as well as after the third dose (Spearman’s ρ=0.59; P<0.0001; Figure 2A). No correlation however was observed between the magnitude of the COVID-19 vaccine antibody response, in terms of serum anti-spike Receptor Binding Domain (RBD) IgG concentrations after either the second or third vaccine dose, and the percentage of spike-specific CD4+ T-cells at those times (Spearman’s ρ = -0.09, P=0.41 for post-second dose; ρ = -0.11, P=0.32 for post-third dose; Figure 2B).
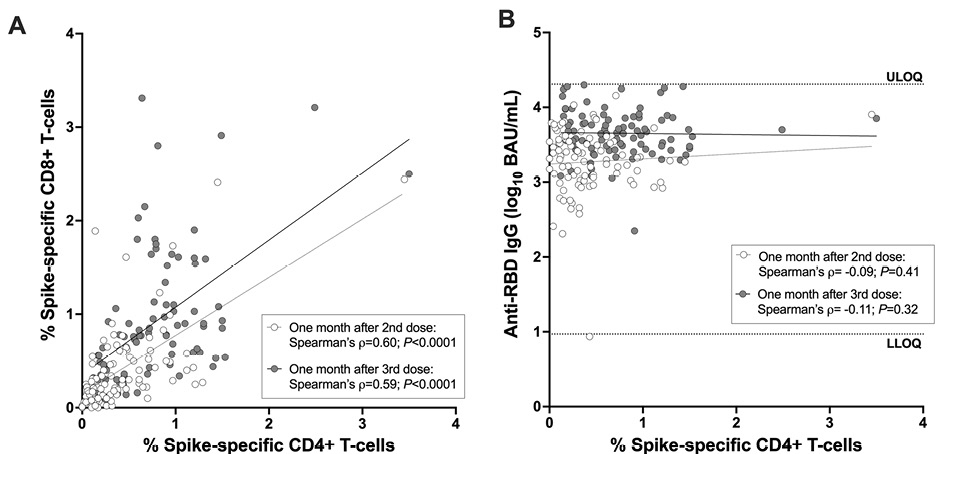
Figure 2. Correlations between cellular and humoral immune measures after 2- and 3-dose COVID-19 mRNA vaccination. Panel A: Correlation between spike-specific CD4+ and CD8+ T-cell frequencies 1 month after the second dose (open circles) and 1 month after the third dose (closed circles) in the combined cohort. Panel B: Correlation between spike-specific CD4+ T-cell frequencies and spike-specific IgG binding antibodies, measured in 1 month after the second dose (open circles) and 1 month after the third dose (closed circles) in the combined cohort. All participants are COVID-19 naive.
Participants expressed a total of 29 different HLA-A, 42 HLA-B, and 23 HLA-C alleles, defined at the subtype level (Supplementary Figure 2). Exploratory analyses that included all HLA alleles observed in a minimum of 5 participants revealed that, following 3 vaccine doses, expression of HLA-A*02:03 was associated with higher spike-specific CD8+ T-cell response frequencies after correcting for multiple comparisons P=0.01; Q=0.08), while expression of B*39:01, B*44:03, or C*07:01 was associated with lower spike-specific CD8+ T-cell response frequencies (all P<0.03, Q<0.13) (Supplementary Table 3). After correcting for multiple comparisons, we did not identify any HLA class I alleles that were significantly associated with spike-specific CD8+ T-cell response frequencies after 2 vaccine doses, but the top allele after 2 vaccine doses was also A*02:03 (P=0.02; Q=0.34; not shown).
To further explore the interaction with A*02:03, we used NetMHCpan-4.1 [25] to identify all 8-, 9-, and 10-amino acid spike epitopes that are predicted to bind to this allele or to the common and closely related A*02:01 allele, which showed no beneficial impact on CD8+ T-cell responses after 3 vaccine doses (P=0.46, Q=0.56; Supplementary Table 3). In total, 29 strong binders (% Rank <0.5) and 56 weak binders (% Rank 0.5-2) were observed for A*02:03, compared to 20 strong binders and 58 weak binders for A*02:01 (Supplementary Table 4). A cumulative view of strong binders, plotted by % Rank, is shown in Figure 3A. An analysis of 30 shared epitopes that displayed strong binding affinity for either allele further revealed that these epitopes were frequently predicted to bind more strongly to A*02:03 than A*02:01 (Wilcoxon matched pairs test, P=0.009) (Figure 3B andSupplementary Table 5). Notably, 7 weak-binding A*02:01 epitopes (all with % Ranks of ~1 to 2) were predicted to bind A*02:03 strongly, while only 1 moderate-binding A*02:03 epitope (GLTVLPPLL, % Rank 0.69) was predicted to bind strongly to A*02:01 (% Rank 0.26) (Figure 3B). These results suggest that higher spike peptide affinity for A*02:03 contributes to enhanced CD8+ T-cell responses following vaccination.
Breakthrough SARS-CoV-2 infections coinciding with the initial waves of Omicron BA.1/BA.2 spread in British Columbia were observed in 8 (20%) OA and 28 (55%) HCW who had received 3 vaccine doses. Our analysis of available PBMC specimens from 24 breakthrough cases (8 OA and 16 HCW) indicated that infection boosted the percentage of both spike-specific CD4+ and CD8+ T-cells (P≤0.008 for all paired longitudinal comparisons; Figure 4A, 4B). The median percentage of spike-specific CD4+ T-cells increased to 0.73% (IQR 0.58-1.10) in OA and 0.86% (IQR 0.51-1.18) in HCW (between-group comparison P=0.78; Figure 4A) while the median percentage of spike-specific CD8+ T-cells increased to 0.98% (IQR 0.81-1.77) in OA and 1.13% (IQR 0.71-1.87) in HCW (between-group comparison P=0.66; Figure 4B). A full longitudinal representation of CD4+ and CD8+ T-cell responses induced by vaccination and breakthrough infection, in all studied participants, is shown in Supplementary Figure 3. Of interest, while the percentage of spike-specific T cells induced by 3 vaccine doses was similar between participants who experienced breakthrough infection (n=36) compared to those who remained COVID-19 naive (n=54) when analyzed altogether (P=0.47 for CD4, P=0.28 for CD8), we found that OA who subsequently became infected (n=8) displayed lower vaccine-induced T-cell responses compared to OA who remained uninfected (n=32) (P=0.03 for CD4, P=0.003 for CD8) (Supplementary Figure 4).
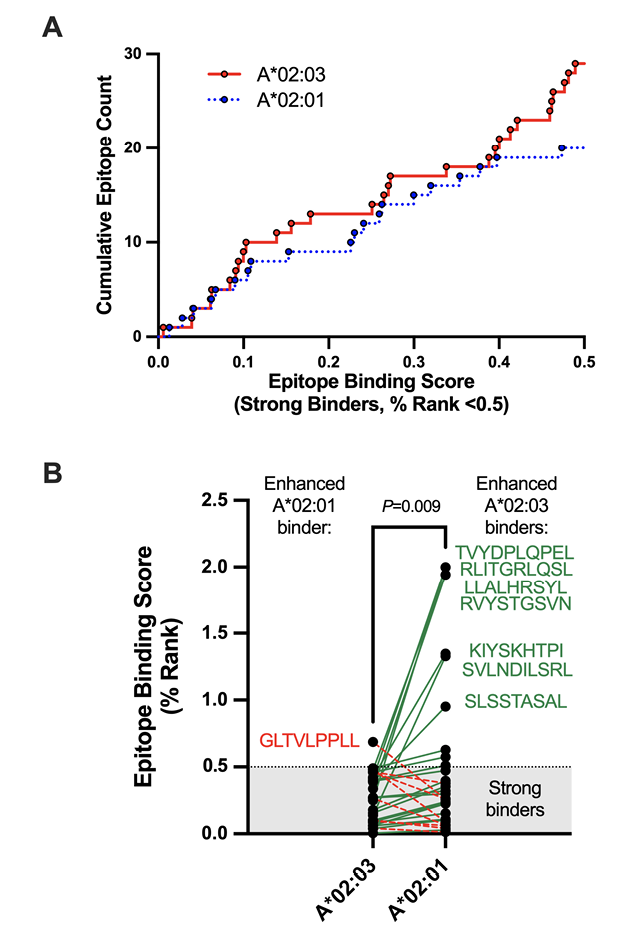
Figure 3. Comparison of HLA-A*02:01 and A*02:03 predicted strong-binding epitopes in SARS-CoV-2 spike. Panel A: Cumulative frequencies of predicted strong-binding epitopes in SARS-CoV-2 spike restricted by HLA-A*02:01 (blue line) and A*02:03. Epitope predictions were performed using NetMHCpan4.1 [25], which defines strong binders as those with % Ranks of 0.5 or lower. The lower the % Rank, the stronger the predicted binding. Panel B: Epitope binding ranks for 30 spike epitopes that were predicted to bind strongly to either A*02:01 or A*02:03. Epitopes that are predicted to bind more strongly to A*02:03 are linked by green lines, with 7 epitopes that were predicted to have the most substantially enhanced A*02:03 binding listed in green next to their corresponding points. Epitopes that are predicted to bind more strongly to A*02:01 are linked by red dotted lines, with one epitope predicted to have the most substantially enhanced A*02:01 binding listed in red next to its corresponding point. The shaded area denotes the threshold for strong binders. P-value computed using the Wilcoxon matched pairs test.
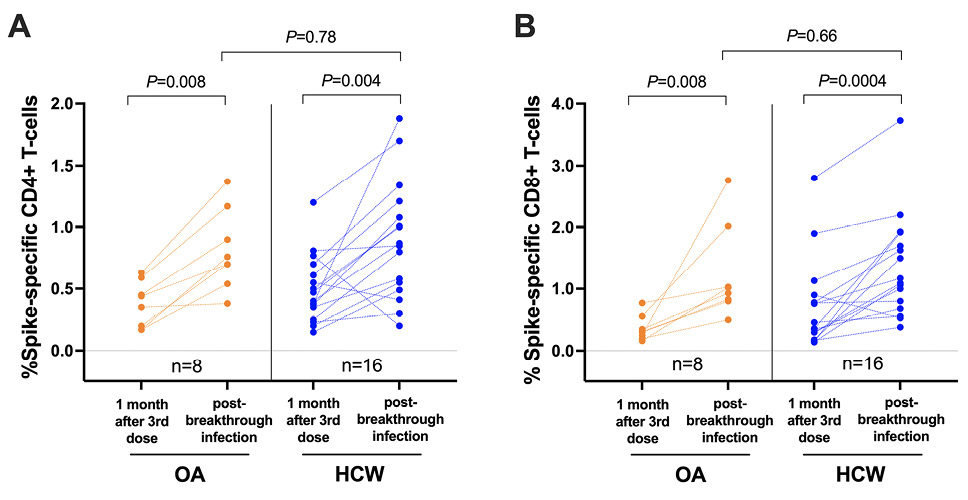
Figure 4. SARS-CoV-2 spike-specific T-cell frequencies in a subset of participants who experienced their first SARS-CoV-2 infection after receiving 3 vaccine doses. Panel A: Spike-specific CD4+ T-cell frequencies in a subset of 8 OA (orange) and 16 HCW (blue) after 3-dose COVID-19 vaccination while participants were still COVID-19-naive, and after a subsequent breakthrough SARS-CoV-2 infection. The Mann-Whitney U-test was used for between-group comparisons and the Wilcoxon matched pairs test was used for longitudinal paired comparisons. P-values are not corrected for multiple comparisons. Panel B: Same as panel A, but for spike-specific CD8+ T-cell responses.
We observed that the frequencies of SARS-CoV-2 spike-specific CD4+ and CD8+ T cells were not significantly different between older and younger adults after 2 doses of COVID-19 mRNA vaccine, and that these responses were enhanced similarly by a third vaccine dose regardless of age. Furthermore, both older and younger adults mounted robust T-cell responses following a post-vaccine breakthrough infection. These results are consistent with a recent report showing that spike-specific T-cell responses measured using an AIM assay were equivalent in older and younger adults after 2 doses of mRNA vaccine [21], although another study reported impaired T-cell effector function, including lower cytokine production, in older adults following 2 vaccine doses [13]. Prior studies have also found weaker T-cell responses in older adults after only 1 vaccine dose [11, 21, 31], but we did not examine this time point here. We also observed no association between CD4+ T-cell responses and RBD-specific IgG antibodies after either 2 or 3 vaccine doses, suggesting that sufficient T-cell help was provided to stimulate spike-specific B-cell responses despite a wide range of antigen-specific CD4+ T-cell frequencies. While our results do not completely rule out the possibility of age-related dysfunction in vaccine-induced T cells among older adults, our data suggest that the impact of any age-associated T-cell impairment is likely to be modest after the second and third vaccine dose.
Our data also indicate that HLA class I alleles modulate the frequency of spike-specific CD8+ T-cell responses following vaccination. Notably, participants expressing HLA-A*02:03 exhibited higher CD8+ T-cell responses, while individuals expressing B*39:01, B*44:03, or C*07:01 exhibited lower responses (although these associations only reached statistical significance after 3 vaccine doses). Bioinformatics analyses predicted a higher number of spike peptides that bound with high affinity to HLA-A*02:03 compared to the closely related A*02:01 allele (which was not associated with a better vaccine response), suggesting that A*02:03 may elicit immunodominant responses to a broader array of vaccine-derived antigens. Several studies have reported associations between HLA genotype and COVID-19 severity or predicted COVID-19 vaccine immunogenicity [32–36], which may underpin ethnicity-related differences in T-cell responses that have been observed in some contexts [37, 38]. Indeed, A*02:03 occurs most frequently in individuals of South and East Asian ancestry, and therefore may contribute to enhanced T-cell responses to COVID-19 vaccines in this population [38]. While we did not observe any impact of either A*02 allele on breakthrough infection frequency in our cohort (data not shown), additional studies are needed to examine these HLA associations in greater detail.
This study has some limitations. First, since this was an observational study, we did not match participant characteristics in the OA and HCW groups at enrollment. Known variables were included during multivariable regression analyses, but we cannot completely rule out the effects of vaccine type (a greater proportion of Moderna doses in OA), ethnicity (a higher frequency of Asian ethnicity in HCW) or other unknown factors on our results. In addition, while the AIM assay provides a very sensitive method to quantify spike-specific CD4+ and CD8+ T cells, we did not analyze T-cell functions such as cytokine production or proliferation that could reveal differences in the effector activity of vaccine- or infection-induced responses. Also, since T-cell responses were measured using peptide pools, we cannot comment on the relative dominance of individual spike epitopes (or host HLAs), nor potential changes in the distribution of these responses over time. We examined T-cell responses using only peptides spanning the ancestral spike protein, which are expected to match the vaccine antigen but may not fully account for responses elicited against SARS-CoV-2 variants following breakthrough infection. Nevertheless, our analysis of spike polymorphisms encoded by Omicron BA.1 suggests that only 4 of the A*02-restricted epitopes were altered in the variant strain, and none of these polymorphisms were predicted to have a significant impact on the strength of peptide binding to A*02:01 or A*02:03 (data not shown). Finally, we did not assess T-cell responses after the first dose of COVID-19 mRNA vaccine, when age-related differences in immune cell frequency or function may be more apparent.
In summary, our study provides evidence that repeated exposure to SARS-CoV-2 spike antigen, either via vaccination or infection, induces steadily higher T-cell frequencies in adults of all ages, at least up to 4 exposures. Importantly, our results also indicate that COVID-19-naive older adults mount robust cellular immune responses after 2- and 3-dose COVID-19 mRNA vaccination, as well as after subsequent breakthrough infection, that are comparable in magnitude to those of younger adults. These results further underscore the benefits of COVID-19 vaccination in the older adult population.
ZLB and MAB led the study. HRL and YS coordinated the study. SD, RK, FM, SS, YS, RW, FY and EB processed specimens and collected data under the supervision of ZLB and MAB. SD, RL, MAB and ZLB analyzed data. MLD, DTH, JS, JSGM and MGR contributed to cohort establishment and/or SARS-CoV-2 serum antibody quantification. SD, ZLB and MAB wrote the manuscript. All authors contributed to manuscript review and editing.
We thank the leadership and staff of Providence Health Care, St. Paul’s Hospital, the BC Centre for Excellence in HIV/AIDS, the Hope to Health Research and Innovation Centre, and Simon Fraser University for supporting this project. We also thank the participants, without whom this study would not have been possible.
This work was supported by the Public Health Agency of Canada through a COVID-19 Immunology Task Force COVID-19 “Hot Spots” Award (2020-HQ-000120 to MGR, ZLB, MAB). Additional funding was received from the Canadian Institutes for Health Research (GA2-177713 and the Coronavirus Variants Rapid Response Network (FRN-175622) to MAB), the Canada Foundation for Innovation through Exceptional Opportunities Fund–COVID-19 awards (to MAB, MLD, ZLB). FM holds a fellowship from the CIHR Canadian HIV Trials Network. FY and EB were supported by an SFU Undergraduate Research Award. MLD and ZLB hold Scholar Awards from the Michael Smith Foundation for Health Research.
The authors report no conflicts of interest to declare.
Supplementary materials are available at the Pathogens and Immunity website. Supplementary data may be provided by the authors to benefit the reader. Supplementary data are not copyedited and are the sole responsibility of the authors. Questions or comments related to supplementary materials should be addressed to the corresponding author.
1. Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-6. doi: 10.1001/jama.2020.4683. PubMed PMID: 32203977.
2. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell C-RC, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052-9. doi: 10.1001/jama.2020.6775. PubMed PMID: 32320003; PMCID: PMC7177629.
3. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-6. Epub 20200708. doi: 10.1038/s41586-020-2521-4. PubMed PMID: 32640463; PMCID: PMC7611074.
4. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-15. Epub 20201210. doi: 10.1056/NEJMoa2034577. PubMed PMID: 33301246; PMCID: PMC7745181.
5. Goronzy JJ, Li G, Yang Z, Weyand CM. The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol. 2013;4:131. Epub 20130604. doi: 10.3389/fimmu.2013.00131. PubMed PMID: 23761790; PMCID: PMC3671290.
6. Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366(9492):1165-74. Epub 20050922. doi: 10.1016/S0140-6736(05)67339-4. PubMed PMID: 16198765.
7. Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373-81. doi: 10.1056/NEJMoa070844. PubMed PMID: 17914038.
8. Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428-36. Epub 20130418. doi: 10.1038/ni.2588. PubMed PMID: 23598398; PMCID: PMC4183346.
9. Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573-83. Epub 20190611. doi: 10.1038/s41577-019-0180-1. PubMed PMID: 31186548; PMCID: PMC7584388.
10. Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Thelwall S, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ladhani SN, Ramsay M, Lopez Bernal J. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N Engl J Med. 2022;386(4):340-50. Epub 20220112. doi: 10.1056/NEJMoa2115481. PubMed PMID: 35021002; PMCID: PMC8781262.
11. Collier DA, Ferreira I, Kotagiri P, Datir RP, Lim EY, Touizer E, Meng B, Abdullahi A, Collaboration C-NBC-, Elmer A, Kingston N, Graves B, Le Gresley E, Caputo D, Bergamaschi L, Smith KGC, Bradley JR, Ceron-Gutierrez L, Cortes-Acevedo P, Barcenas-Morales G, Linterman MA, McCoy LE, Davis C, Thomson E, Lyons PA, McKinney E, Doffinger R, Wills M, Gupta RK. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417-22. Epub 20210630. doi: 10.1038/s41586-021-03739-1. PubMed PMID: 34192737; PMCID: PMC8373615.
12. Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, Rubin C, Freedman L, Kreiss Y, Regev-Yochay G. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385(24):e84. Epub 20211006. doi: 10.1056/NEJMoa2114583. PubMed PMID: 34614326; PMCID: PMC8522797.
13. Palacios-Pedrero MA, Jansen JM, Blume C, Stanislawski N, Jonczyk R, Molle A, Hernandez MG, Kaiser FK, Jung K, Osterhaus A, Rimmelzwaan GF, Saletti G. Signs of immunosenescence correlate with poor outcome of mRNA COVID-19 vaccination in older adults. Nat Aging. 2022;2(10):896-905. Epub 20221014. doi: 10.1038/s43587-022-00292-y. PubMed PMID: 37118289; PMCID: PMC10154205.
14. Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Yaseen F, Kalikawe R, Datwani S, Burns L, Young L, Leung V, Ennis S, Brumme CJ, Montaner JSG, Dong W, Prystajecky N, Lowe CF, DeMarco ML, Holmes D, Simons J, Niikura M, Romney MG, Brumme ZL, Brockman MA. Impact of age and SARS-CoV-2 breakthrough infection on humoral immune responses after three doses of COVID-19 mRNA vaccine. Open Forum Infectious Diseases. 2023; Accepted, In Press.
15. Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Yaseen F, Umviligihozo G, Kalikawe R, Datwani S, Omondi FH, Burns L, Young L, Leung V, Agafitei O, Ennis S, Dong W, Basra S, Lim LY, Ng K, Pantophlet R, Brumme CJ, Montaner JSG, Prystajecky N, Lowe CF, DeMarco ML, Holmes DT, Simons J, Niikura M, Romney MG, Brumme ZL, Brockman MA. Older Adults Mount Less Durable Humoral Responses to Two Doses of COVID-19 mRNA Vaccine but Strong Initial Responses to a Third Dose. J Infect Dis. 2022;226(6):983-94. Epub 2022/05/12. doi: 10.1093/infdis/jiac199. PubMed PMID: 35543278; PMCID: PMC9129202.
16. US Food and Drug Administration. FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations. fda.gov2021.
17. Bowman E. A CDC Panel Backs Booster Shots For Older Adults, A Step Toward Making Them Available. ‘National Public Radio, USA. 2021 23 Sept 2021.
18. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186-93. Epub 20220201. doi: 10.1038/s41590-021-01122-w. PubMed PMID: 35105982.
19. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, Baxter AE, Herati RS, Oldridge DA, Gouma S, Hicks P, Dysinger S, Lundgreen KA, Kuri-Cervantes L, Adamski S, Hicks A, Korte S, Giles JR, Weirick ME, McAllister CM, Dougherty J, Long S, D’Andrea K, Hamilton JT, Betts MR, Bates P, Hensley SE, Grifoni A, Weiskopf D, Sette A, Greenplate AR, Wherry EJ. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54(9):2133-42 e3. Epub 20210813. doi: 10.1016/j.immuni.2021.08.001. PubMed PMID: 34453880; PMCID: PMC8361141.
20. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, Brachtendorf S, Lorks V, Sikorski J, Hilker R, Becker D, Eller AK, Grutzner J, Boesler C, Rosenbaum C, Kuhnle MC, Luxemburger U, Kemmer-Bruck A, Langer D, Bexon M, Bolte S, Kariko K, Palanche T, Fischer B, Schultz A, Shi PY, Fontes-Garfias C, Perez JL, Swanson KA, Loschko J, Scully IL, Cutler M, Kalina W, Kyratsous CA, Cooper D, Dormitzer PR, Jansen KU, Tureci O. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586(7830):594-9. Epub 20200930. doi: 10.1038/s41586-020-2814-7. PubMed PMID: 32998157.
21. Jo N, Hidaka Y, Kikuchi O, Fukahori M, Sawada T, Aoki M, Yamamoto M, Nagao M, Morita S, Nakajima TE, Muto M, Hamazaki Y. Impaired CD4(+) T cell response in older adults is associated with reduced immunogenicity and reactogenicity of mRNA COVID-19 vaccination. Nat Aging. 2023;3(1):82-92. Epub 20230112. doi: 10.1038/s43587-022-00343-4. PubMed PMID: 37118516; PMCID: PMC10154196.
22. Brockman MA, Mwimanzi F, Lapointe HR, Sang Y, Agafitei O, Cheung PK, Ennis S, Ng K, Basra S, Lim LY, Yaseen F, Young L, Umviligihozo G, Omondi FH, Kalikawe R, Burns L, Brumme CJ, Leung V, Montaner JSG, Holmes D, DeMarco ML, Simons J, Pantophlet R, Niikura M, Romney MG, Brumme ZL. Reduced Magnitude and Durability of Humoral Immune Responses to COVID-19 mRNA Vaccines Among Older Adults. J Infect Dis. 2022;225(7):1129-40. Epub 2021/12/11. doi: 10.1093/infdis/jiab592. PubMed PMID: 34888688; PMCID: PMC8689804.
23. Brumme ZL, Brumme CJ, Chui C, Mo T, Wynhoven B, Woods CK, Henrick BM, Hogg RS, Montaner JS, Harrigan PR. Effects of human leukocyte antigen class I genetic parameters on clinical outcomes and survival after initiation of highly active antiretroviral therapy. J Infect Dis. 2007;195(11):1694-704. Epub 20070424. doi: 10.1086/516789. PubMed PMID: 17471440.
24. Woods CK, Brumme CJ, Liu TF, Chui CK, Chu AL, Wynhoven B, Hall TA, Trevino C, Shafer RW, Harrigan PR. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol. 2012;50(6):1936-42. Epub 20120307. doi: 10.1128/JCM.06689-11. PubMed PMID: 22403431; PMCID: PMC3372133.
25. Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48(W1):W449-W54. doi: 10.1093/nar/gkaa379. PubMed PMID: 32406916; PMCID: PMC7319546.
26. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440-5. Epub 20030725. doi: 10.1073/pnas.1530509100. PubMed PMID: 12883005; PMCID: PMC170937.
27. Skowronski DM, Febriani Y, Ouakki M, Setayeshgar S, El Adam S, Zou M, Talbot D, Prystajecky N, Tyson JR, Gilca R, Brousseau N, Deceuninck G, Galanis E, Fjell CD, Sbihi H, Fortin E, Barkati S, Sauvageau C, Naus M, Patrick DM, Henry B, Hoang LMN, De Wals P, Garenc C, Carignan A, Drolet M, Jassem AN, Sadarangani M, Brisson M, Krajden M, De Serres G. Two-Dose Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Effectiveness With Mixed Schedules and Extended Dosing Intervals: Test-Negative Design Studies From British Columbia and Quebec, Canada. Clin Infect Dis. 2022;75(11):1980-92. doi: 10.1093/cid/ciac290. PubMed PMID: 35438175; PMCID: PMC9047203.
28. Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasreen S, Calzavara A, Lu D, Harris TM, Yu K, Wilson SE. Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. JAMA Netw Open. 2022;5(6):e2218505. Epub 20220601. doi: 10.1001/jamanetworkopen.2022.18505. PubMed PMID: 35749115; PMCID: PMC9233237.
29. Organization WH. Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19. 2021 Updated 18 August 2022. Report No.
30. BC Centre for Disease Control. Weekly update on Variants of Concern 2022. Available from: http://www.bccdc.ca/health-info/diseases-conditions/covid-19/data.
31. Muller L, Andree M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, Ostermann PN, Robitzsch R, Hauka S, Walker A, Menne C, Grutza R, Timm J, Adams O, Schaal H. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin Infect Dis. 2021;73(11):2065-72. Epub 2021/04/28. doi: 10.1093/cid/ciab381. PubMed PMID: 33906236; PMCID: PMC8135422.
32. Bose T, Pant N, Pinna NK, Bhar S, Dutta A, Mande SS. Does immune recognition of SARS-CoV2 epitopes vary between different ethnic groups? Virus Res. 2021;305:198579. Epub 20210921. doi: 10.1016/j.virusres.2021.198579. PubMed PMID: 34560183; PMCID: PMC8453877.
33. Rao V, Chandra N. In-silico study of influence of HLA heterogeneity on CTL responses across ethnicities to SARS-CoV-2. Hum Immunol. 2022;83(12):797-802. Epub 20221011. doi: 10.1016/j.humimm.2022.09.008. PubMed PMID: 36229378; PMCID: PMC9550298.
34. Bertinetto FE, Magistroni P, Mazzola GA, Costa C, Elena G, Alizzi S, Scozzari G, Migliore E, Galassi C, Ciccone G, Ricciardelli G, Scarmozzino A, Angelone L, Cassoni P, Cavallo R, Vaisitti T, Deaglio S, Amoroso A, Collaborative G. The humoral and cellular response to mRNA SARS-CoV-2 vaccine is influenced by HLA polymorphisms. HLA. 2023. Epub 20230403. doi: 10.1111/tan.15049. PubMed PMID: 37010080.
35. Srivastava A, Hollenbach JA. The immunogenetics of COVID-19. Immunogenetics. 2023;75(3):309-20. Epub 20221219. doi: 10.1007/s00251-022-01284-3. PubMed PMID: 36534127; PMCID: PMC9762652.
36. Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, Kaplan IM, Alehashemi S, Burbelo PD, Bhuyan F, de Jesus AA, Dobbs K, Rosen LB, Cheng A, Shaw E, Vakkilainen MS, Pala F, Lack J, Zhang Y, Fink DL, Oikonomou V, Snow AL, Dalgard CL, Chen J, Sellers BA, Montealegre Sanchez GA, Barron K, Rey-Jurado E, Vial C, Poli MC, Licari A, Montagna D, Marseglia GL, Licciardi F, Ramenghi U, Discepolo V, Lo Vecchio A, Guarino A, Eisenstein EM, Imberti L, Sottini A, Biondi A, Mato S, Gerstbacher D, Truong M, Stack MA, Magliocco M, Bosticardo M, Kawai T, Danielson JJ, Hulett T, Askenazi M, Hu S, Group NIRtC, Chile MISCG, Pavia Pediatric C-G, Cohen JI, Su HC, Kuhns DB, Lionakis MS, Snyder TM, Holland SM, Goldbach-Mansky R, Tsang JS, Notarangelo LD. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. 2022;28(5):1050-62. Epub 20220217. doi: 10.1038/s41591-022-01724-3. PubMed PMID: 35177862; PMCID: PMC9119950.
37. Smith M, Abdesselem HB, Mullins M, Tan TM, Nel AJM, Al-Nesf MAY, Bensmail I, Majbour NK, Vaikath NN, Naik A, Ouararhni K, Mohamed-Ali V, Al-Maadheed M, Schell DT, Baros-Steyl SS, Anuar ND, Ismail NH, Morris PE, Mamat RNR, Rosli NSM, Anwar A, Ellan K, Zain RM, Burgers WA, Mayne ES, El-Agnaf OMA, Blackburn JM. Age, Disease Severity and Ethnicity Influence Humoral Responses in a Multi-Ethnic COVID-19 Cohort. Viruses. 2021;13(5). Epub 20210428. doi: 10.3390/v13050786. PubMed PMID: 33925055; PMCID: PMC8146997.
38. Martin CA, Nazareth J, Jarkhi A, Pan D, Das M, Logan N, Scott S, Bryant L, Abeywickrama N, Adeoye O, Ahmed A, Asif A, Bandi S, George N, Gohar M, Gray LJ, Kaszuba R, Mangwani J, Martin M, Moorthy A, Renals V, Teece L, Vail D, Khunti K, Moss P, Tattersall A, Hallis B, Otter AD, Rowe C, Willett BJ, Haldar P, Cooper A, Pareek M. Ethnic differences in cellular and humoral immune responses to SARS-CoV-2 vaccination in UK healthcare workers: a cross-sectional analysis. EClinicalMedicine. 2023;58:101926. Epub 20230404. doi: 10.1016/j.eclinm.2023.101926. PubMed PMID: 37034357; PMCID: PMC10071048.
Submitted July 25, 2023 | Accepted September 18, 2023 | Published November 17, 2023
Copyright © 2023 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.