Lela Kardava1,#, Clarisa M. Buckner1,#, and Susan Moir1
1 Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD
#Contributed equally to this work
Susan Moir
Laboratory of Immunoregulation, NIAID, NIH
smoir@niaid.nih.gov
Kardava L, Buckner CM, Moir S. B-Cell Responses to Sars-Cov-2 mRNA Vaccines. Pathogens and Immunity. 2022;7(2):93–119. doi: 10.20411/pai.v7i2.550.
10.20411/pai.v7i2.550
Most vaccines against viral pathogens protect through the acquisition of immunological memory from long-lived plasma cells that produce antibodies and memory B cells that can rapidly respond upon an encounter with the pathogen or its variants. The COVID-19 pandemic and rapid deployment of effective vaccines have provided an unprecedented opportunity to study the immune response to a new yet rapidly evolving pathogen. Here we review the scientific literature and our efforts to understand antibody and B-cell responses to SARS-CoV-2 vaccines, the effect of SARS-CoV-2 infection on both primary and secondary immune responses, and how repeated exposures may impact outcomes.
SARS-CoV-2; vaccination; protective immunity; B cells; plasmablasts; memory response; antibodies
The rapid global spread of SARS-CoV-2 and its deadly consequences quickly mobilized the scientific community to develop a panoply of vaccines [1], several of which have been highly effective at preventing transmission or severe disease [2]. While the COVID-19 pandemic has caused worldwide suffering that will be felt for years to come, it has provided a few silver linings, one being an unprecedented opportunity to learn how the human immune system responds to a new pathogen and how new technologies can be rapidly translated into safe and effective vaccines. As with most vaccines, protection from SARS-CoV-2 infection or COVID-19 illness is largely mediated by antibodies, in this case against the viral spike protein delivered in the form of mRNA-based nanoparticles, adenoviral vectors, inactivated whole virus, or recombinant proteins [3]. Humoral immunity is sustained by 2 distinct arms of the B lineage, the plasma cells that secrete the antibodies and the memory B cells (MBCs) that can rapidly differentiate into antibody-secreting cells upon re-encounter with antigen. Antibody-mediated immunity can also be divided into different categories: namely primary versus secondary or recall responses, direct antigen-neutralizing and protective non-neutralizing functions, and whether a response or phase of a response is independent or not of T-cell help. This review will mainly focus on human B cells that are involved in generating and sustaining protective immunity following exposure to SARS-CoV-2 through infection and/or vaccination. Excellent reviews can be found elsewhere on the functional aspects of antibodies [4], and lineages such as B-1 and marginal zone B cells (MZB) involved in providing T-independent natural immunity [5]. This review takes a chronological approach both in terms of the different stages of the COVID-19 pandemic and how they relate to different stages of B-cell immunity.
In the early days of the COVID-19 pandemic when death rates were high, it quickly became apparent that the host response to the virus could have both disease exacerbating and beneficial effects [6–9]. Among the earliest B cells to be detected in the peripheral blood after acute infection or vaccination are plasmablasts, actively proliferating low affinity antibody-secreting cells that arise during the early extrafollicular T-independent phase of the immune response [5]. Plasmablasts are among the earliest indicators of acute infection, including with SARS-CoV-2 [10–12], in part because they are rapidly induced after exposure but also because basal levels are low and their distinctive features make their appearance in the circulation easy to track. The surge in plasmablasts may also arise from antigen nonspecific innate responses involving cytokines that are triggered by acute viral infections, including SARS-CoV-2 [13]. This acute cytokine storm includes IL-6, CXCL10, and IL-10 [14], which can induce B-cell terminal differentiation, even in the absence of antigen [15]. In HIV infection, serum levels of these cytokines correlate with frequencies of plasmablasts in the blood, of which the highest frequencies occur during early infection when viremia is high [16]. In SARS-CoV-2 infection, the immunoglobulin gene repertoire of acute-phase plasmablasts has been reported as diverse, suggesting a polyclonal rather than virus-specific induction [10]. However, in other studies, plasmablast expansions have been associated with high antiviral antibody titers suggestive of a specific response to the virus [17, 18]. The plasmablasts may contribute to the early low-maturity antibody response that declines rapidly before a more stable neutralizing response arises from long-lived bone marrow-derived plasma cells [19]. However, there is uncertainty regarding the longevity of plasma cells against SARS-CoV-2 [20], especially given the evidence of rapidly waning immunity following infection [21].
The rapid development and approval of vaccines against SARS-CoV-2 under the emergency authorization use in late 2020 provided an unprecedented opportunity to investigate immune responses to what was essentially a neoantigen and to do so with study designs that could include baseline analyses which could not be performed with SARS-CoV-2 infection. This was also the first opportunity to delineate responses to an mRNA-based vaccine in humans. For the purpose of this review, the 2-dose regimen of either the mRNA-1273 (Moderna) or the BNT162b2 (Pfizer-BioNTech) is considered a primary immunization. As with SARS-CoV-2 infection, plasmablasts are induced rapidly following vaccination, of which a fraction may reflect recall response to cross-reactive elements in the S2 subunit of coronavirus spike proteins [22]. However, in contrast to infection or other chronic conditions where plasmablasts in the blood can remain elevated for as long as the active disease persists [23], those induced by vaccination tend to arise and disappear rapidly, consistent with their short-lived nature [24, 25]. In response to a first exposure, vaccine-specific plasmablasts in the blood reach their peak nearly 2 weeks after vaccination [26], while for subsequent exposures, the peak occurs faster, within about 7 days, and the plasmablasts are more likely to originate from an MBC recall response [27]. However, plasmablast responses can vary with different types of vaccines and routes of administration [28]. A further confounder is that most studies report on total plasmablasts and not necessarily those that are specific to the vaccine.
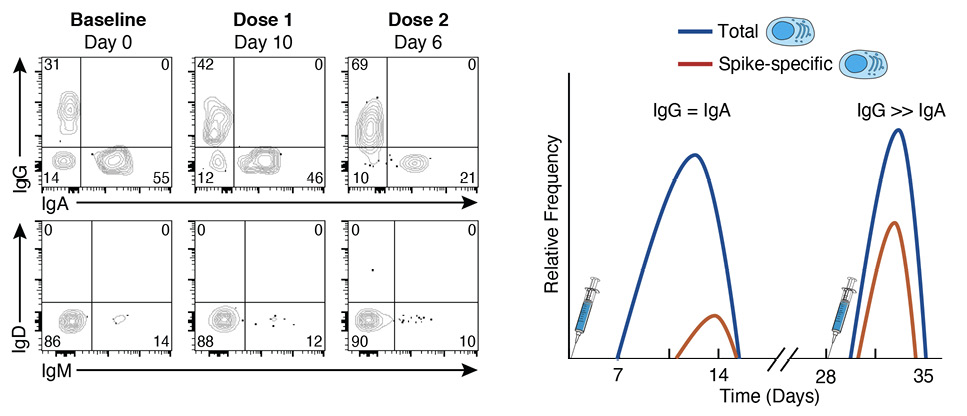
When SARS-CoV-2 vaccines became available at the end of 2020, NIH employees who were eligible received their vaccines onsite, which happened to be the 2-dose mRNA-1273 vaccine, given 28 days apart [3]. We took this opportunity to evaluate the kinetics and magnitude of the B-cell and antibody response to this novel vaccine in a cohort of 21 NIH healthcare and laboratory employees. We used spectral flow cytometry, which captures the entire emissions spectrum and generates a spectral fingerprint for each fluorochrome [29], to evaluate both antigen-specific (the SARS-CoV-2 spike protein) and non-specific B-cell responses among the major B-cell subsets that circulate in the peripheral blood, including plasmablasts, on freshly isolated samples [30]. We use the term non-specific to define responses that are not antigen-specified and note that a portion of this response may be specific but not captured by the assay used. Our design overcame 2 common challenges when studying plasmablasts: 1) the unavoidable detrimental effects of cryopreservation on their viability [31]; and 2) the inherent limitations of quantifying antigen-specific plasmablasts using quasi single parameter methods such as ELISPOT [32]. Our design also had its limitations, including the drift in lasers and settings that are difficult to normalize when performing assays in real-time over an extended period and the reduced surface expression of the B-cell receptor (BCR) that occurs as B cells differentiate into plasmablasts and increase their expression of immunoglobulins (Igs), which are destined for secretion [33]. The loss of cell surface BCR expression on plasmablasts is more pronounced for IgG than IgA, the predominant isotype at steady state (Figure 1). There is also evidence that the ratio of surface and intracellular expression of Igs differs with conditions and tissue origin [33]. Nonetheless, we and others have used flow cytometry to identify and quantify antigen-specific plasmablasts without the need to perform intracellular staining [27, 30, 34].

Figure 1. Plasmablast responses to SARS-CoV-2 vaccination. The flow cytometry plots are representative of immunoglobulin isotype cell-surface staining of plasmablasts at baseline and at day 10 after the first dose and day 6 after the second dose of the mRNA-1273 vaccine. The plots illustrate that IgA is the dominant isotype at baseline (steady state) while IgG dominates after vaccination, IgM remains low and stable over time, and the cumulative distribution of the 3 isotypes is ~100% at each timepoint. The graphical depiction on the right illustrates the kinetics for total (antigen non-specific) and spike-specific plasmablasts following doses 1 and 2 of the mRNA-1273 vaccine. The non-specific response precedes the spike-specific response after dose 1, while both are elicited intensely albeit more transiently after dose 2 and dominated by IgG for both spike-specific and non-specific responses.
Several approaches can be taken to verify the experimental design; in our study we confirmed that ~95% of all plasmablasts had an accounted Ig isotype [30], IgA, IgG, or IgM (Figure 1). Verification also included the comparison of 2 methods of plasmablast quantification, one based on flow cytometry and the other by ELISPOT, as well as the comparison of isotype distribution with and without permeabilization [30].
Having determined that infection or vaccine-induced SARS-CoV-2-specific plasmablasts in the blood could be quantified by spectral flow cytometry, we proceeded with an unbiased and integrated approach (explained in detail below) to longitudinally evaluate primary antibody, plasmablast, and MBC responses to the 2-dose mRNA-1273 in our cohort of 21 SARS-CoV-2-naive healthy adults [30]. A detectable rise in total (SARS-CoV-2 spike nonspecific) IgG and IgA plasmablasts was observed approximately 7 days after the first vaccine dose and peaked around day 10 (illustrated in Figure 1 and clusters C9 and C13 respectively in Figure 2). Plasmablasts that were specific for SARS-CoV-2 spike protein could be detected by day 10 and peaked after day 14, the last timepoint evaluated after dose 1 [30]. Both levels of spike-specific and non-specific plasmablasts had returned to baseline when the second dose was administered at day 28. The plasmablast response to the second dose was rapid, intense, and short-lived, with a peak occurring 5-7 days after vaccination (illustrated in Figure 1). We then addressed whether vaccine-induced plasmablasts measured in the peripheral blood were predictive of the antibody response measured in the serum. We found spike non-specific plasmablasts at days 10 and 14 after dose 1, and spike-specific plasmablasts at days 7 and 10 after dose 2 were correlated with both IgG and IgA antibody titers measured at 2 and 6 months after vaccination [30]. While few studies have established similar correlations, it may be because most studies have been performed on cryopreserved cells at relatively few timepoints. The observation that frequencies of circulating plasmablasts are predictors of the antibody response to SARS-CoV-2 vaccination could reflect overall immune competency and/or that plasmablasts are precursors of plasma cells that home to the bone marrow and secrete the antibodies, which are then measured in serum [35]. Recent findings on the dynamics of influenza vaccine-induced B-cell and antibody responses provide evidence for a direct link between plasmablasts, bone marrow plasma cells, and serum antibodies [36].
While the concept of immunological memory, the ability to rapidly respond to a previously encountered pathogen through the generation of affinity-matured MBCs, is straightforward, the reality of identifying and tracking MBCs is far more challenging. Since the identification almost 25 years ago of CD27 as the canonical or conventional marker of human MBCs [37, 38], several phenotypically distinct populations of MBCs have been identified [39]. Their numbers and relative distribution can vary with time, condition, and anatomical location, all of which suggest that the heterogeneity of MBCs also reflects distinct functionalities in response to pathogens [40, 41]. With the development of new technologies and computational tools that can handle the acquisition and analysis of large datasets [29], several recent studies have provided new insights into the complexity and diversity of B-cell populations, as well as clarity regarding MBCs involved in the generation of immunological memory [31, 42]. For example, while the existence of MBCs that lack expression of CD27 has been known for some time [43–45], only recently have combinations of markers such as CD200, CD45RB, CD11a, and CD11c helped discriminate between the various conventional and nonconventional human MBCs [31, 42]. When combined with analyses of BCR mutational burden, integrated approaches can also provide insight into the positioning of various MBC populations along a maturational trajectory, such as the progressive accumulation of mutations from CD27-CD45RB- to CD27-CD45RB+ to CD27+CD45RB-/+ MBCs [31]. However, while much progress has been made, certain markers may have a more nuanced profile than has been assumed from past experience. For example, MBCs are often defined as CD38- or CD38lo [31, 46]. However, while transitional B cells and plasmablasts express distinctly high levels of CD38 and all naive B cells express intermediate levels of CD38, MBCs can express a wide range of CD38 intensities, from the very low intensities on nonconventional (CD11c+CD21loCD27+/-) MBCs to variable yet distinct intensities on conventional (CD11c-CD21+CD27+) MBCs [30, 47]. Another caution is using the BCR mutational burden to assess the maturational stage of an MBC population that has a heterogeneous or partially undefined Ig isotype profile, especially given that Ig isotype is a strong determinant of mutational burden [48]. In the example given above, MBCs, defined by expression of CD27 and CD45RB, had different distributions of Ig isotype which could have contributed to the differences in mutational burdens reported [31]. Nonetheless, integrated multi-omic approaches are providing new means of classifying B cells that will help advance our understanding of MBCs, how they are generated and how they contribute to immunological memory.
In our flow cytometric approach to evaluating B-cell signatures of the primary antibody response to the 2-dose SARS-CoV-2 mRNA-1273 vaccine, we designed a panel that included markers for distinguishing between conventional and non-conventional MBCs (CD11c, CD21, and CD27), as well as the major Ig isotypes (IgD, IgM, IgG, and IgA). The reasoning for including all 4 Ig isotypes was 2-fold: to compare cellular Ig isotypes with their corresponding spike-binding antibody titers and to extend analyses of manually defined populations to unsupervised clustering that would capture a broad spectrum of phenotypes [30]. Given the potential pitfalls of using dimensional reduction and clustering algorithms to generate immune signatures [29], the inclusion of the 4 major Ig isotypes provided a means of verifying clusters based on canonical rules. The most abundant clusters should be naive B cells expressing higher intensities of IgD than IgM, and there should be few and minimally abundant clusters that contain incompatible isotypes; for example, a cluster should not contain both unswitched (IgD/M) and switched (IgG or IgA) isotypes or IgG with IgA. We also found that a 30-cluster setting was ideal for generating a wide spectrum of distinct phenotypes without compromising visual clarity or quantitative analyses. Having ascertained that our clustering met these metrics and was consistent with manually defined populations, we then evaluated the temporal dynamics of each cluster in response to the 2 vaccine doses by measuring fluctuations of each cluster (the non-specific response) and of spike-bound cells (the antigen-specific response) within each cluster [30]. This approach identified several MBC clusters that fluctuated over time after each vaccine dose, and a number of these clusters were found to correlate with antibody titers at months 2 and 6 post-vaccination. An early spike-specific correlate of both timepoints was cluster 6 (C6; illustrated in Figure 2), an IgG-switched CD38+ MBC lacking expression of CD27 that has been described as an early-response MBC [49]. Notably, frequencies at baseline of dose 2 of spike-specific cells in C6 correlated with the IgG and IgA antibody responses at months 2 and 6 post-vaccination. One might ask why an IgG-expressing MBC like C6 would correlate with IgA antibodies? It is likely that these correlates are indicative of an overall responsiveness rather than a cause-effect relationship. In this regard, C6 was among several clusters, both switched and unswitched MBCs, that were early (dose 1) antigen non-specific correlates of the IgG spike-specific antibody response [30].
Our study on B-cell signatures of antibody response to mRNA vaccination included several MBC subsets. Among the 30 clusters generated by the unsupervised analysis, 18 were MBCs, consistent with their heterogeneous nature and a reflection of the B-cell markers chosen to study the MBC response. Among the 18 MBC clusters, 9 were classified as conventional, as defined by MBCs that express both CD21 and CD27, then further annotated as unswitched or IgA or IgG switched, and as being either CD38+ or CD38-, consistent with another recent study [47]. The remaining 9 MBC clusters were classified as nonconventional, either because they lacked expression of CD27 or had profiles associated with activation (CD20hiCD21loCD11c+). Among these were MBCs we have previously described in the context of persistent HIV viremia and named activated (CD27+) or tissue-like (CD27-) [45], although the term atypical is now commonly used to refer to both collectively [50]. Atypical MBCs accounted for 6 clusters: either unswitched or IgA or IgG switched, and among each, they were further delineated by the expression of CD27 [30]. Notably, none of these atypical MBCs expressed CD38, consistent with previous reporting [51]. Several of the atypical MBC clusters underwent spike-specific and non-specific fluctuations following vaccination. However, while several atypical MBC clusters correlated antigen-nonspecifically with antibody titers at month 2, few were spike-specific correlates, despite one of these clusters, C5, an IgG-expressing atypical MBC comprising a large fraction of the spike-specific MBC response days 7-14 after dose 2 [30], and depicted in Figure 2. Thus, it was the non-specific overall activation of atypical MBCs that was predictive of the antibody response. In contrast, a strong spike-specific MBC predictor of antibody responses at both months 2 and 6 post-vaccination was C2 (Figure 2), an IgG-expressing resting MBC that also accounted for most of the strong month 6 spike-specific MBC response that we and others have described [30, 52]. However, one important caveat to our study is that our panel did not include the activation marker CD71, which is strongly induced following vaccination or infection [27]. It will be important to include this marker in future studies as it seems to have a different expression profile than other markers of activation, such as CD11c and CD95 [31].
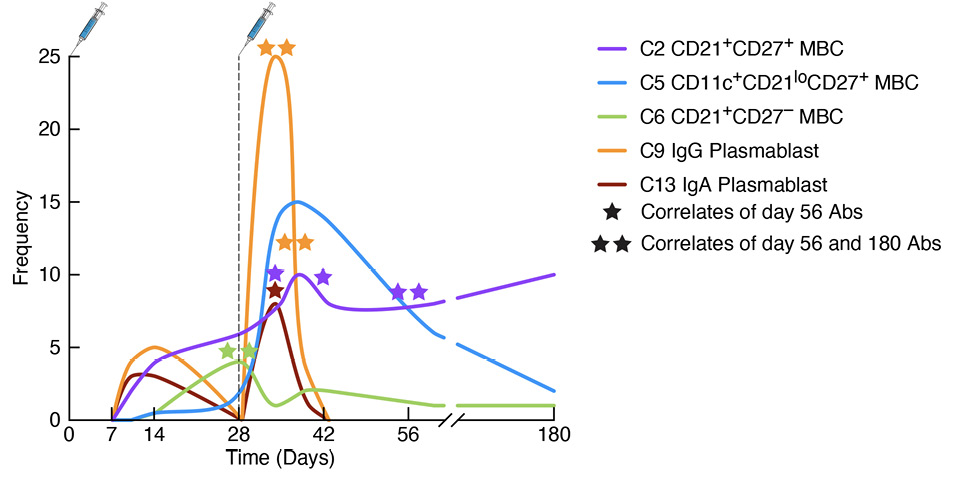
Figure 2. Kinetics of spike-specific plasmablasts and memory B-cell responses to SARS-CoV-2 vaccination. The graph depicts cellular responses over time following doses 1 and 2 of the mRNA-1273 vaccine, with corresponding cluster (C) designations and annotations discussed in the review. The stars refer to the timepoints when the indicated spike-specific plasmablast or memory B-cell (MBC) cluster correlated with either day 56/month 2 (1 star) or both day 56/month 2 and day 180/month 6 (2 stars) of the spike-specific antibody response. Both plasmablast clusters correlated with the antibody response, as did the early MBC C6 and the conventional MBC C2 but not atypical MBC C5. Abs, antibodies.
Since the start of the COVID-19 pandemic in 2020, exposures to SARS-CoV-2 have transitioned in rapid succession from primary infection and vaccination alone to secondary combined exposures, and most recently, to multiple combinations of re-infection and/or booster vaccination. Given the high transmissibility of the virus and the rapid development and distribution, albeit uneven, of vaccines, the number of people worldwide who remain unexposed to SARS-CoV-2 has been quickly diminishing. This means that there are fewer and fewer people who remain immunologically naive to the virus and conversely, there is a growing number of people with diverse types of exposures, whether from infection and/or vaccination. These changing dynamics pose both challenges and opportunities; while it is becoming increasingly difficult to understand how multiple and varied exposures shape the immune response to SARS-CoV-2, the knowledge gained may help understand what sustains protection against rapidly evolving viruses such as SARS-CoV-2. Furthermore, much of the knowledge with SARS-CoV-2 is likely to be applicable to other current and future viral pathogens.
The term hybrid refers to the type of immunity that arises from a combination of infection and vaccination, in either order. While the definition is simple, the reality of tracking and understanding hybrid immunity is far from simple. For example, should we consider infection with other coronaviruses as part of hybrid immunity given the evidence that immune responses to SARS-CoV-2 can be modulated by cross-reactive B-cell and T-cell responses that were acquired prior to the pandemic [22, 53]? Exposure histories are important because they can influence the magnitude and longevity of subsequent immune responses yet are affected by factors and mechanisms that are not well understood. Contributing factors likely include the nature and timing of each exposure, and the role of imprinting, the process by which the immune system tends to amplify a response to the first version of the pathogen encountered at the expense of subsequent variants [54]. We tackle a few of these factors here.
The first reports on the effects of prior infection on the immune response to vaccination began to emerge in the summer of 2021, approximately 6 months after the first SARS-CoV-2 vaccines received emergency use approval [55]. Several studies performed during this period demonstrated that 1 or 2 doses of the mRNA vaccines augmented the magnitude and durability of neutralizing antibody titers and increased breadth among serum and memory B-cell derived antibodies in people who were previously infected when compared to infected/unvaccinated or uninfected/vaccinated individuals [56–65]. Of note, however, the second dose was found to provide minimal enhancement of antibody and B-cell responses over the first dose in previously infected individuals [58], contrasting with a strong response to a second dose in uninfected individuals [57, 62, 65]. These findings were early indications that the benefit provided by hybrid immunity may be restricted by factors associated with time interval or repeated exposure, as has been shown with other vaccines and in animal models [66–68]. A large retrospective study addressing the risk of infection also found that a second dose of the BNT162b2 mRNA vaccine in people who were previously infected did not increase protection from re-infection over the first dose [69]. Regarding time interval between doses, several studies on cohorts that have included both uninfected and previously infected individuals have shown that an extended interval between the first 2 doses of the mRNA vaccines increases the magnitude and breadth of B-cell and/or antibody responses when compared to the standard 3-to-4-week interval [62, 70–75]. However, while immunological benefits of an extended dosing interval have clearly been demonstrated, evidence for protection from infection or severe disease is more nuanced. In another large retrospective study demonstrating enhanced protection from vaccination after prior infection, a prolonged interval between vaccine doses did not contribute to added protection in either uninfected or previously infected participants [76]. However, other, albeit smaller and less definitive studies have demonstrated increased protection with extended intervals [73, 77], consistent with health-impact modeling [78], and changes in guidelines on dosing interval [79]. Several factors may contribute to differences in predicted or observed outcomes, including demographics, period of study, circulating variants, levels and sources of pre-existing immunity, as well as differences in vaccine efficacy [78, 80].
As the number of exposures to SARS-CoV-2, either from vaccination, infection, or combinations of these increases, one factor that should be considered in trying to understand the cumulative effect of exposures on immunity and protection is whether the source (vaccine or virus) of the exposure matters. In the early phase of the pandemic, clinical studies on the 2-dose mRNA vaccine regimens showed similar or higher titers of antibodies in vaccinees when compared to convalescent controls [81, 82]. Subsequent epidemiological studies suggested that protection from infection was superior after vaccination than infection [83, 84]. However, more recent studies have shown the reverse, that protection from infection is greater from a previous infection than from vaccination alone [85–88]. This reversal may reflect the changing nature of the pandemic and the different therapies and modalities used to treat people with COVID-19, although the risks of poor outcomes from infection continue to far outweigh those from vaccination [89]. It should also be noted that recovery from natural infection has at least 2 inherent advantages over current SARS-CoV-2 vaccines: a longer period of persisting antigen that can drive greater affinity maturation and breadth of MBCs [90], and the induction of an early, more robust and possibly more effective IgA mucosal response [91, 92].
As vaccination rates have increased, studies have shifted from considering effects from either infection or vaccination alone on immunity or protection to focusing on how the combination of infection and vaccination compares to vaccination or infection alone, although the latter is increasingly rare. Several large epidemiological studies have shown that protection from symptomatic infection is highest among people who were previously infected and vaccinated [76, 87, 93, 94]. A synergistic effect involving imprinted cellular responses may explain the enhanced magnitude of antibody responses and breadth for protection against emerging variants that has been described with hybrid immunity [95, 96]. However, the benefits from hybrid immunity may diminish as the number of vaccine doses increases. Studies have shown that infection followed by 1 dose of the mRNA vaccine induces higher frequencies of spike-specific plasmablasts and/or MBCs compared to 2 doses of vaccine alone [52, 59, 97]. However, as mentioned above, antibody and B-cell responses to a second vaccine dose did not increase over the first dose in people who were previously infected [57, 58, 62, 65]. Notably, differences in frequencies of spike-specific MBCs between uninfected and prior-infected diminished 2-3 months after vaccination [97], and by month 6 after vaccination, frequencies of spike-specific MBCs and MBC responses to variants of concern were similar between previously infected and uninfected vaccinees [52]. However, one advantage of hybrid immunity may be the level of somatic hypermutation, which at 3-4 months post-vaccination was higher in the prior-infected than uninfected group [52]. Other studies have shown similar outcomes [59, 95]; however, one caveat is that these analyses were performed at relatively early timepoints after vaccination.
The limited benefit of the second dose of an mRNA vaccine in previously infected individuals may be due to repeated exposure to antigen and/or the short time interval between doses. While repeated exposure to antigen under controlled conditions has been shown to induce potent, broad, and sustained immunity [98], these conditions are difficult to control and can lead to suboptimal responses. Antibodies from a previous exposure can mediate feedback inhibition by limiting antigen access during germinal center reactions [99], and repeated antigen exposure can create bottlenecks and restrict antibody diversity [66]. Diminished antibody responses to influenza vaccination have been linked to repeated vaccination and high pre-existing antibody titers [67, 68]. A similar mechanism may also be restricting B-cell responses to SARS-CoV-2 vaccines in people who have received passive administration of SARS-CoV-2 antibodies [100]. Furthermore, atypical MBCs, which are induced by SARS-CoV-2 infection and vaccination [30, 97], have been shown under conditions of chronic antigen stimulation to over-express inhibitory receptors and diminish their responsiveness to further stimulation [45]. In a recent study on malaria, it was proposed that while atypical MBCs are induced following exposure to the pathogen through infection or vaccination, repeated boosting favors atypical over conventional MBCs [101]. Thus, the outcome of MBC recall responses in the context of repeated exposures remains difficult to predict, posing challenges to the goals of generating sustained protective immunity against current and future SARS-CoV-2 variants and other pathogens [102, 103].
We recently evaluated the effect of infection on antibody and B-cell responses to a booster (third) dose of the mRNA vaccines in a cohort including people who were infected prior to or after vaccination or who remained uninfected throughout the 2-month study period [104]. Similar to other studies [34], we found that a third dose in uninfected individuals induced robust and broad antibody and B-cell responses that were increased in people infected post-boost yet muted in those who were infected prior to receiving their booster vaccine. The antibody and B-cell responses induced were most restricted in those individuals who received their vaccine closest to their time of infection. This abrogation of antibody and B-cell responses to a third dose of mRNA vaccine by recent SARS-CoV-2 infection is consistent with a recent report at 7 days post-vaccination [105], and the blunted responses observed after the second dose in previously infected people [57, 58, 62, 65].
Several possibilities could explain the unresponsiveness of B cells to a third vaccine dose after a recent SARS-CoV-2 infection. First, we considered anergy or exhaustion that has been described for CD21lo B cells (reviewed in [54]), which are expanded during SARS-CoV-2 infection [18, 96, 97]. Under conditions of chronic activation, these CD21lo B cells show signs of activation in vivo yet are refractory to further stimulation ex vivo [106], a phenomenon that has been termed post-activated anergy or exhaustion [107]. A hallmark of anergic B cells is failure to respond to BCR stimulation, as measured by calcium flux or phosphorylation of signaling molecules [108]. We focused on spleen tyrosine kinase (SYK) and the downstream phospholipase Cg2 (pPLCg2), 2 signaling molecules that undergo coordinated and rapid phosphorylation following BCR stimulation yet have profiles that distinguish different MBC subsets [31], and become dysregulated in severe cases of SARS-CoV-2 due to decreased expression of inhibitory receptors [109]. When we evaluated the response to stimulation among SARS-CoV-2 recently infected and uninfected individuals, we found that BCR-mediated signaling among spike-specific MBCs was higher at baseline in the individuals who were previously infected when compared to those who were uninfected, although levels were similar between the 2 groups at day 60 post-vaccination. Furthermore, we found that the fold difference in BCR signaling between baseline and day 60 was correlated with the interval between infection and vaccination [104].
The differences we observed in B-cell signaling between prior-infected and uninfected individuals were at baseline, prompting us to consider whether this reflected differences in phenotypes of spike-specific MBCs at this timepoint. While we did not find that baseline frequencies of spike-specific CD21lo atypical MBCs differed between prior-infected and uninfected groups, we did find a significantly higher frequency of CD21+CD27lo MBCs in the uninfected group [104]. Notably, these MBCs have been associated with a durable response to influenza vaccination [110], and more recently to SARS-CoV-2 vaccination [111, 112]. They are also similar in phenotype to the early MBC C6 that we found to correlate with primary immunization antibody responses [30] and depicted in Figure 2. In the recent study, we found that CD21+CD27lo MBCs were less responsive to BCR stimulation than their CD21+CD27+ counterpart, providing an explanation for why baseline B-cell signaling was higher in the prior-infected group where CD21+CD27+ MBCs were enriched [104]. We do not have a mechanistic explanation for the differences in BCR signaling and there may be other factors contributing to the muted response of B cells to a third vaccine dose in people who were recently infected with SARS-CoV-2. Our findings do not address whether these differences were driven by infection per se or any source of repeated antigenic stimulation. Nonetheless, our findings may help provide guidance on when booster vaccines should be administered.
The primary immune response to SARS-CoV-2 mRNA vaccines engages both plasmablasts and MBCs that can be evaluated simultaneously using high-dimensional flow cytometry. Given the heterogeneity of the responding B cells and their fluctuations over time, longitudinal studies that track immune responses should be performed using analytical tools that can allow for maximum uniformity and meaningful discovery. While there is a growing number of methods and algorithms being developed for projecting and clustering large sets of data, these applications are only useful if the flow cytometry is performed adequately. Beyond these considerations, there remains ongoing challenges of describing and discussing cluster-based complex datasets in ways that are concise and informative. Secondary immune responses to SARS-CoV-2 are best evaluated by considering exposures from both infection and vaccination. While it is clear that hybrid immunity generated from both infection and vaccination is superior to either alone, there is mounting evidence that repeated exposures may restrict antibody and B-cell responses. This evidence comes from studies that revealed muted immune responses and limited protection from a second dose of the primary 2-dose mRNA vaccines in people who were previously infected, and recent findings of a similarly muted response to booster vaccines. However, many of these observations have been based on small studies and clearly there is more to learn on the effect of timing between vaccine doses and the various factors that can impact immunity and protection against SARS-CoV-2 infection or re-infection.
The authors declare no conflicts of interest.
SM wrote the manuscript. LK and CMB edited and contributed intellectual content to the manuscript. All authors read and approved the final version.
This work was funded by the Intramural Research Program of the Division of Intramural Research, NIAID, NIH.
1. Corey L, Mascola JR, Fauci AS, Collins FS. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948-50. doi: 10.1126/science.abc5312. PubMed PMID: 32393526.
2. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861-80. doi: 10.1016/j.cell.2021.01.007. PubMed PMID: 33497610; PMCID: PMC7803150.
3. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475-84. doi: 10.1038/s41577-021-00578-z. PubMed PMID: 34211186; PMCID: PMC8246128.
4. Goldblatt D, Alter G, Crotty S, Plotkin SA. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev. 2022;310(1):6-26. doi: 10.1111/imr.13091. PubMed PMID: 35661178; PMCID: PMC9348242.
5. Baumgarth N. The Shaping of a B Cell Pool Maximally Responsive to Infections. Annu Rev Immunol. 2021;39:103-29. doi: 10.1146/annurev-immunol-042718-041238. PubMed PMID: 33472004.
6. Mohanraj D, Whitelegg A. Trilogy of COVID-19: Infection, Vaccination, and Immunosuppression. Int Arch Allergy Immunol. 2022;183(8):888-906. doi: 10.1159/000524056. PubMed PMID: 35390803; PMCID: PMC9148894.
7. Mistry P, Barmania F, Mellet J, Peta K, Strydom A, Viljoen IM, James W, Gordon S, Pepper MS. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front Immunol. 2021;12:809244. doi: 10.3389/fimmu.2021.809244. PubMed PMID: 35046961; PMCID: PMC8761766.
8. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122-7. doi: 10.1126/science.abm8108. PubMed PMID: 35271343.
9. Carsetti R, Quinti I, Locatelli F. COVID-19 - pathogenesis and immunological findings across the clinical manifestation spectrum. Curr Opin Pulm Med. 2021;27(3):193-8. doi: 10.1097/MCP.0000000000000775. PubMed PMID: 33629970.
10. Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, Blish CA. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat Med. 2020;26(7):1070-6. doi: 10.1038/s41591-020-0944-y. PubMed PMID: 32514174; PMCID: PMC7382903.
11. Bernardes JP, Mishra N, Tran F, Bahmer T, Best L, Blase JI, Bordoni D, Franzenburg J, Geisen U, Josephs-Spaulding J, Kohler P, Kunstner A, Rosati E, Aschenbrenner AC, Bacher P, Baran N, Boysen T, Brandt B, Bruse N, Dorr J, Drager A, Elke G, Ellinghaus D, Fischer J, Forster M, Franke A, Franzenburg S, Frey N, Friedrichs A, Fuss J, Gluck A, Hamm J, Hinrichsen F, Hoeppner MP, Imm S, Junker R, Kaiser S, Kan YH, Knoll R, Lange C, Laue G, Lier C, Lindner M, Marinos G, Markewitz R, Nattermann J, Noth R, Pickkers P, Rabe KF, Renz A, Rocken C, Rupp J, Schaffarzyk A, Scheffold A, Schulte-Schrepping J, Schunk D, Skowasch D, Ulas T, Wandinger KP, Wittig M, Zimmermann J, Busch H, Hoyer BF, Kaleta C, Heyckendorf J, Kox M, Rybniker J, Schreiber S, Schultze JL, Rosenstiel P, Network HCALB, Deutsche C-OI. Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity. 2020;53(6):1296-314 e9. doi: 10.1016/j.immuni.2020.11.017. PubMed PMID: 33296687; PMCID: PMC7689306.
12. Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, Piechocka-Trocha A, Lefteri K, Osborn M, Bals J, Bartsch YC, Bonheur N, Caradonna TM, Chevalier J, Chowdhury F, Diefenbach TJ, Einkauf K, Fallon J, Feldman J, Finn KK, Garcia-Broncano P, Hartana CA, Hauser BM, Jiang C, Kaplonek P, Karpell M, Koscher EC, Lian X, Liu H, Liu J, Ly NL, Michell AR, Rassadkina Y, Seiger K, Sessa L, Shin S, Singh N, Sun W, Sun X, Ticheli HJ, Waring MT, Zhu AL, Alter G, Li JZ, Lingwood D, Schmidt AG, Lichterfeld M, Walker BD, Yu XG, Padera RF, Jr., Pillai S, Massachusetts Consortium on Pathogen Readiness Specimen Working G. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell. 2020;183(1):143-57 e13. doi: 10.1016/j.cell.2020.08.025. PubMed PMID: 32877699; PMCID: PMC7437499.
13. Mangalmurti N, Hunter CA. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53(1):19-25. doi: 10.1016/j.immuni.2020.06.017. PubMed PMID: 32610079; PMCID: PMC7321048.
14. Tay SH, Toh MMX, Thian YL, Vellayappan BA, Fairhurst AM, Chan YH, Aminkeng F, Bharwani LD, Huang Y, Mak A, Wong ASC. Cytokine Release Syndrome in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Case Series of 25 Patients and Review of the Literature. Front Immunol. 2022;13:807050. doi: 10.3389/fimmu.2022.807050. PubMed PMID: 35154124; PMCID: PMC8831742.
15. Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127(3):701-21 e1-70. doi: 10.1016/j.jaci.2010.11.050. PubMed PMID: 21377040.
16. Buckner CM, Moir S, Ho J, Wang W, Posada JG, Kardava L, Funk EK, Nelson AK, Li Y, Chun TW, Fauci AS. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol. 2013;87(10):5800-11. doi: 10.1128/JVI.00094-13. PubMed PMID: 23487459; PMCID: PMC3648153.
17. Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, Agyekum RS, Mathew D, Baxter AE, Vella LA, Kuthuru O, Apostolidis SA, Bershaw L, Dougherty J, Greenplate AR, Pattekar A, Kim J, Han N, Gouma S, Weirick ME, Arevalo CP, Bolton MJ, Goodwin EC, Anderson EM, Hensley SE, Jones TK, Mangalmurti NS, Luning Prak ET, Wherry EJ, Meyer NJ, Betts MR. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5(49). doi: 10.1126/sciimmunol.abd7114. PubMed PMID: 32669287; PMCID: PMC7402634.
18. Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, Ley AM, Kyu S, Howell JC, Ozturk T, Lee S, Suryadevara N, Case JB, Bugrovsky R, Chen W, Estrada J, Morrison-Porter A, Derrico A, Anam FA, Sharma M, Wu HM, Le SN, Jenks SA, Tipton CM, Staitieh B, Daiss JL, Ghosn E, Diamond MS, Carnahan RH, Crowe JE, Jr., Hu WT, Lee FE, Sanz I. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020;21(12):1506-16. doi: 10.1038/s41590-020-00814-z. PubMed PMID: 33028979; PMCID: PMC7739702.
19. Turner JS, Kim W, Kalaidina E, Goss CW, Rauseo AM, Schmitz AJ, Hansen L, Haile A, Klebert MK, Pusic I, O’Halloran JA, Presti RM, Ellebedy AH. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421-5. doi: 10.1038/s41586-021-03647-4. PubMed PMID: 34030176.
20. Nguyen DC, Lamothe PA, Woodruff MC, Saini AS, Faliti CE, Sanz I, Lee FE. COVID-19 and plasma cells: Is there long-lived protection? Immunol Rev. 2022;309(1):40-63. doi: 10.1111/imr.13115. PubMed PMID: 35801537; PMCID: PMC9350162.
21. Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, Davenport MP. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21(6):395-404. doi: 10.1038/s41577-021-00550-x. PubMed PMID: 33927374; PMCID: PMC8082486.
22. Amanat F, Thapa M, Lei T, Ahmed SMS, Adelsberg DC, Carreno JM, Strohmeier S, Schmitz AJ, Zafar S, Zhou JQ, Rijnink W, Alshammary H, Borcherding N, Reiche AG, Srivastava K, Sordillo EM, van Bakel H, Personalized Virology I, Turner JS, Bajic G, Simon V, Ellebedy AH, Krammer F. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell. 2021;184(15):3936-48 e10. doi: 10.1016/j.cell.2021.06.005. PubMed PMID: 34192529; PMCID: PMC8185186.
23. Jenks SA, Cashman KS, Woodruff MC, Lee FE, Sanz I. Extrafollicular responses in humans and SLE. Immunol Rev. 2019;288(1):136-48. doi: 10.1111/imr.12741. PubMed PMID: 30874345; PMCID: PMC6422038.
24. Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat Rev Immunol. 2019;19(6):383-97. doi: 10.1038/s41577-019-0143-6. PubMed PMID: 30837674.
25. Elsner RA, Shlomchik MJ. Germinal Center and Extrafollicular B Cell Responses in Vaccination, Immunity, and Autoimmunity. Immunity. 2020;53(6):1136-50. doi: 10.1016/j.immuni.2020.11.006. PubMed PMID: 33326765; PMCID: PMC7748291.
26. Kohler S, Bethke N, Bothe M, Sommerick S, Frentsch M, Romagnani C, Niedrig M, Thiel A. The early cellular signatures of protective immunity induced by live viral vaccination. Eur J Immunol. 2012;42(9):2363-73. doi: 10.1002/eji.201142306. PubMed PMID: 22733156.
27. Ellebedy AH, Jackson KJ, Kissick HT, Nakaya HI, Davis CW, Roskin KM, McElroy AK, Oshansky CM, Elbein R, Thomas S, Lyon GM, Spiropoulou CF, Mehta AK, Thomas PG, Boyd SD, Ahmed R. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat Immunol. 2016;17(10):1226-34. doi: 10.1038/ni.3533. PubMed PMID: 27525369; PMCID: PMC5054979.
28. Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, Kasturi S, Carlone GM, Quinn C, Chaussabel D, Palucka AK, Mulligan MJ, Ahmed R, Stephens DS, Nakaya HI, Pulendran B. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15(2):195-204. doi: 10.1038/ni.2789. PubMed PMID: 24336226; PMCID: PMC3946932.
29. Cossarizza A, Chang HD, Radbruch A, Abrignani S, Addo R, Akdis M, Andra I, Andreata F, Annunziato F, Arranz E, Bacher P, Bari S, Barnaba V, Barros-Martins J, Baumjohann D, Beccaria CG, Bernardo D, Boardman DA, Borger J, Bottcher C, Brockmann L, Burns M, Busch DH, Cameron G, Cammarata I, Cassotta A, Chang Y, Chirdo FG, Christakou E, Cicin-Sain L, Cook L, Corbett AJ, Cornelis R, Cosmi L, Davey MS, De Biasi S, De Simone G, Del Zotto G, Delacher M, Di Rosa F, Di Santo J, Diefenbach A, Dong J, Dorner T, Dress RJ, Dutertre CA, Eckle SBG, Eede P, Evrard M, Falk CS, Feuerer M, Fillatreau S, Fiz-Lopez A, Follo M, Foulds GA, Frobel J, Gagliani N, Galletti G, Gangaev A, Garbi N, Garrote JA, Geginat J, Gherardin NA, Gibellini L, Ginhoux F, Godfrey DI, Gruarin P, Haftmann C, Hansmann L, Harpur CM, Hayday AC, Heine G, Hernandez DC, Herrmann M, Hoelsken O, Huang Q, Huber S, Huber JE, Huehn J, Hundemer M, Hwang WYK, Iannacone M, Ivison SM, Jack HM, Jani PK, Keller B, Kessler N, Ketelaars S, Knop L, Knopf J, Koay HF, Kobow K, Kriegsmann K, Kristyanto H, Krueger A, Kuehne JF, Kunze-Schumacher H, Kvistborg P, Kwok I, Latorre D, Lenz D, Levings MK, Lino AC, Liotta F, Long HM, Lugli E, MacDonald KN, Maggi L, Maini MK, Mair F, Manta C, Manz RA, Mashreghi MF, Mazzoni A, McCluskey J, Mei HE, Melchers F, Melzer S, Mielenz D, Monin L, Moretta L, Multhoff G, Munoz LE, Munoz-Ruiz M, Muscate F, Natalini A, Neumann K, Ng LG, Niedobitek A, Niemz J, Almeida LN, Notarbartolo S, Ostendorf L, Pallett LJ, Patel AA, Percin GI, Peruzzi G, Pinti M, Pockley AG, Pracht K, Prinz I, Pujol-Autonell I, Pulvirenti N, Quatrini L, Quinn KM, Radbruch H, Rhys H, Rodrigo MB, Romagnani C, Saggau C, Sakaguchi S, Sallusto F, Sanderink L, Sandrock I, Schauer C, Scheffold A, Scherer HU, Schiemann M, Schildberg FA, Schober K, Schoen J, Schuh W, Schuler T, Schulz AR, Schulz S, Schulze J, Simonetti S, Singh J, Sitnik KM, Stark R, Starossom S, Stehle C, Szelinski F, Tan L, Tarnok A, Tornack J, Tree TIM, van Beek JJP, van de Veen W, van Gisbergen K, Vasco C, Verheyden NA, von Borstel A, Ward-Hartstonge KA, Warnatz K, Waskow C, Wiedemann A, Wilharm A, Wing J, Wirz O, Wittner J, Yang JHM, Yang J. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur J Immunol. 2021;51(12):2708-3145. doi: 10.1002/eji.202170126. PubMed PMID: 34910301.
30. Kardava L, Rachmaninoff N, Lau WW, Buckner CM, Trihemasava K, Blazkova J, Lopes de Assis F, Wang W, Zhang X, Wang Y, Chiang CI, Narpala S, McCormack GE, Liu C, Seamon CA, Sneller MC, O’Connell S, Li Y, McDermott AB, Chun TW, Fauci AS, Tsang JS, Moir S. Early human B cell signatures of the primary antibody response to mRNA vaccination. Proc Natl Acad Sci USA. 2022;119(28):e2204607119. doi: 10.1073/pnas.2204607119. PubMed PMID: 35759653; PMCID: PMC9282446.
31. Glass DR, Tsai AG, Oliveria JP, Hartmann FJ, Kimmey SC, Calderon AA, Borges L, Glass MC, Wagar LE, Davis MM, Bendall SC. An Integrated Multi-omic Single-Cell Atlas of Human B Cell Identity. Immunity. 2020;53(1):217-32 e5. doi: 10.1016/j.immuni.2020.06.013. PubMed PMID: 32668225; PMCID: PMC7369630.
32. Carter MJ, Mitchell RM, Meyer Sauteur PM, Kelly DF, Truck J. The Antibody-Secreting Cell Response to Infection: Kinetics and Clinical Applications. Front Immunol. 2017;8:630. doi: 10.3389/fimmu.2017.00630. PubMed PMID: 28620385; PMCID: PMC5451496.
33. Mei HE, Yoshida T, Sime W, Hiepe F, Thiele K, Manz RA, Radbruch A, Dorner T. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood. 2009;113(11):2461-9. doi: 10.1182/blood-2008-04-153544. PubMed PMID: 18987362.
34. Goel RR, Painter MM, Lundgreen KA, Apostolidis SA, Baxter AE, Giles JR, Mathew D, Pattekar A, Reynaldi A, Khoury DS, Gouma S, Hicks P, Dysinger S, Hicks A, Sharma H, Herring S, Korte S, Kc W, Oldridge DA, Erickson RI, Weirick ME, McAllister CM, Awofolaju M, Tanenbaum N, Dougherty J, Long S, D’Andrea K, Hamilton JT, McLaughlin M, Williams JC, Adamski S, Kuthuru O, Drapeau EM, Davenport MP, Hensley SE, Bates P, Greenplate AR, Wherry EJ. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185(11):1875-87 e8. doi: 10.1016/j.cell.2022.04.009. PubMed PMID: 35523182; PMCID: PMC8989683.
35. Seifert M, Kuppers R. Human memory B cells. Leukemia. 2016;30(12):2283-92. doi: 10.1038/leu.2016.226. PubMed PMID: 27499139.
36. Davis CW, Jackson KJL, McCausland MM, Darce J, Chang C, Linderman SL, Chennareddy C, Gerkin R, Brown SJ, Wrammert J, Mehta AK, Cheung WC, Boyd SD, Waller EK, Ahmed R. Influenza vaccine-induced human bone marrow plasma cells decline within a year after vaccination. Science. 2020;370(6513):237-41. doi: 10.1126/science.aaz8432. PubMed PMID: 32792465.
37. Tangye SG, Liu YJ, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188(9):1691-703. doi: 10.1084/jem.188.9.1691. PubMed PMID: 9802981; PMCID: PMC2212517.
38. Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188(9):1679-89. doi: 10.1084/jem.188.9.1679. PubMed PMID: 9802980; PMCID: PMC2212515.
39. Sanz I, Wei C, Jenks SA, Cashman KS, Tipton C, Woodruff MC, Hom J, Lee FE. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front Immunol. 2019;10:2458. doi: 10.3389/fimmu.2019.02458. PubMed PMID: 31681331; PMCID: PMC6813733.
40. Akkaya M, Kwak K, Pierce SK. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol. 2020;20(4):229-38. doi: 10.1038/s41577-019-0244-2. PubMed PMID: 31836872; PMCID: PMC7223087.
41. Cyster JG, Allen CDC. B Cell Responses: Cell Interaction Dynamics and Decisions. Cell. 2019;177(3):524-40. doi: 10.1016/j.cell.2019.03.016. PubMed PMID: 31002794; PMCID: PMC6538279.
42. Weisel NM, Joachim SM, Smita S, Callahan D, Elsner RA, Conter LJ, Chikina M, Farber DL, Weisel FJ, Shlomchik MJ. Surface phenotypes of naive and memory B cells in mouse and human tissues. Nat Immunol. 2022;23(1):135-45. doi: 10.1038/s41590-021-01078-x. PubMed PMID: 34937918; PMCID: PMC8712407.
43. Fecteau JF, Cote G, Neron S. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol. 2006;177(6):3728-36. doi: 10.4049/jimmunol.177.6.3728. PubMed PMID: 16951333.
44. Ehrhardt GR, Hsu JT, Gartland L, Leu CM, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202(6):783-91. doi: 10.1084/jem.20050879. PubMed PMID: 16157685; PMCID: PMC2212938.
45. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797-805. doi: 10.1084/jem.20072683. PubMed PMID: 18625747; PMCID: PMC2525604.
46. Carsetti R, Corrente F, Capponi C, Mirabella M, Cascioli S, Palomba P, Bertaina V, Pagliara D, Colucci M, Piano Mortari E. Comprehensive phenotyping of human peripheral blood B lymphocytes in pathological conditions. Cytometry A. 2022;101(2):140-9. doi: 10.1002/cyto.a.24518. PubMed PMID: 34851033; PMCID: PMC9299869.
47. Liechti T, Iftikhar Y, Mangino M, Beddall M, Goss CW, O’Halloran JA, Mudd PA, Roederer M. Immune phenotypes that are associated with subsequent COVID-19 severity inferred from post-recovery samples. Nat Commun. 2022 Nov 25;13(1):7255. doi:10.1038/s41467-022-34638-2. PMID: 36433939.
48. Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, He B, Biermann K, Lange JF, van der Burg M, van Dongen JJ, van Zelm MC. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118(8):2150-8. doi: 10.1182/blood-2011-04-345579. PubMed PMID: 21690558; PMCID: PMC3342861.
49. Grimsholm O, Piano Mortari E, Davydov AN, Shugay M, Obraztsova AS, Bocci C, Marasco E, Marcellini V, Aranburu A, Farroni C, Silvestris DA, Cristofoletti C, Giorda E, Scarsella M, Cascioli S, Barresi S, Lougaris V, Plebani A, Cancrini C, Finocchi A, Moschese V, Valentini D, Vallone C, Signore F, de Vincentiis G, Zaffina S, Russo G, Gallo A, Locatelli F, Tozzi AE, Tartaglia M, Chudakov DM, Carsetti R. The Interplay between CD27(dull) and CD27(bright) B Cells Ensures the Flexibility, Stability, and Resilience of Human B Cell Memory. Cell Rep. 2020;30(9):2963-77 e6. doi: 10.1016/j.celrep.2020.02.022. PubMed PMID: 32130900.
50. Portugal S, Obeng-Adjei N, Moir S, Crompton PD, Pierce SK. Atypical memory B cells in human chronic infectious diseases: An interim report. Cell Immunol. 2017;321:18-25. doi: 10.1016/j.cellimm.2017.07.003. PubMed PMID: 28735813; PMCID: PMC5732066.
51. Austin JW, Buckner CM, Kardava L, Wang W, Zhang X, Melson VA, Swanson RG, Martins AJ, Zhou JQ, Hoehn KB, Fisk JN, Dimopoulos Y, Chassiakos A, O’Dell S, Smelkinson MG, Seamon CA, Kwan RW, Sneller MC, Pittaluga S, Doria-Rose NA, McDermott A, Li Y, Chun TW, Kleinstein SH, Tsang JS, Petrovas C, Moir S. Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation. Sci Transl Med. 2019;11(520). doi: 10.1126/scitranslmed.aax0904. PubMed PMID: 31776286; PMCID: PMC7479651.
52. Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, Lundgreen KA, Reynaldi A, Khoury DS, Pattekar A, Gouma S, Kuri-Cervantes L, Hicks P, Dysinger S, Hicks A, Sharma H, Herring S, Korte S, Baxter AE, Oldridge DA, Giles JR, Weirick ME, McAllister CM, Awofolaju M, Tanenbaum N, Drapeau EM, Dougherty J, Long S, D’Andrea K, Hamilton JT, McLaughlin M, Williams JC, Adamski S, Kuthuru O, dagger UPCPU, Frank I, Betts MR, Vella LA, Grifoni A, Weiskopf D, Sette A, Hensley SE, Davenport MP, Bates P, Luning Prak ET, Greenplate AR, Wherry EJ. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374(6572):abm0829. doi: 10.1126/science.abm0829. PubMed PMID: 34648302; PMCID: PMC9284784.
53. Scheid JF, Barnes CO, Eraslan B, Hudak A, Keeffe JR, Cosimi LA, Brown EM, Muecksch F, Weisblum Y, Zhang S, Delorey T, Woolley AE, Ghantous F, Park SM, Phillips D, Tusi B, Huey-Tubman KE, Cohen AA, Gnanapragasam PNP, Rzasa K, Hatziioanno T, Durney MA, Gu X, Tada T, Landau NR, West AP, Jr., Rozenblatt-Rosen O, Seaman MS, Baden LR, Graham DB, Deguine J, Bieniasz PD, Regev A, Hung D, Bjorkman PJ, Xavier RJ. B cell genomics behind cross-neutralization of SARS-CoV-2 variants and SARS-CoV. Cell. 2021;184(12):3205-21 e24. doi: 10.1016/j.cell.2021.04.032. PubMed PMID: 34015271; PMCID: PMC8064835.
54. McGrath JJC, Li L, Wilson PC. Memory B cell diversity: insights for optimized vaccine design. Trends Immunol. 2022;43(5):343-54. doi: 10.1016/j.it.2022.03.005. PubMed PMID: 35393268; PMCID: PMC8977948.
55. Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54(8):1636-51. doi: 10.1016/j.immuni.2021.07.017. PubMed PMID: 34348117; PMCID: PMC8328682.
56. Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, Hoffmann HH, Barnes CO, Cipolla M, Ramos V, Oliveira TY, Cho A, Schmidt F, Da Silva J, Bednarski E, Aguado L, Yee J, Daga M, Turroja M, Millard KG, Jankovic M, Gazumyan A, Zhao Z, Rice CM, Bieniasz PD, Caskey M, Hatziioannou T, Nussenzweig MC. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595(7867):426-31. doi: 10.1038/s41586-021-03696-9. PubMed PMID: 34126625; PMCID: PMC8277577.
57. Goel RR, Apostolidis SA, Painter MM, Mathew D, Pattekar A, Kuthuru O, Gouma S, Hicks P, Meng W, Rosenfeld AM, Dysinger S, Lundgreen KA, Kuri-Cervantes L, Adamski S, Hicks A, Korte S, Oldridge DA, Baxter AE, Giles JR, Weirick ME, McAllister CM, Dougherty J, Long S, D’Andrea K, Hamilton JT, Betts MR, Luning Prak ET, Bates P, Hensley SE, Greenplate AR, Wherry EJ. Distinct antibody and memory B cell responses in SARS-CoV-2 naive and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58). doi: 10.1126/sciimmunol.abi6950. PubMed PMID: 33858945; PMCID: PMC8158969.
58. Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, Neradilek M, Seydoux E, Jennewein MF, MacCamy AJ, Feng J, Mize G, De Rosa SC, Finzi A, Lemos MP, Cohen KW, Moodie Z, McElrath MJ, McGuire AT. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021. doi: 10.1126/science.abg9175. PubMed PMID: 33766944; PMCID: PMC8139425.
59. Sokal A, Broketa M, Barba-Spaeth G, Meola A, Fernandez I, Fourati S, Azzaoui I, de La Selle A, Vandenberghe A, Roeser A, Bouvier-Alias M, Crickx E, Languille L, Michel M, Godeau B, Gallien S, Melica G, Nguyen Y, Zarrouk V, Canoui-Poitrine F, Noizat-Pirenne F, Megret J, Pawlotsky JM, Fillatreau S, Simon-Loriere E, Weill JC, Reynaud CA, Rey FA, Bruhns P, Chappert P, Mahevas M. Analysis of mRNA vaccination-elicited RBD-specific memory B cells reveals strong but incomplete immune escape of the SARS-CoV-2 Omicron variant. Immunity. 2022;55(6):1096-104 e4. doi: 10.1016/j.immuni.2022.04.002. PubMed PMID: 35483354; PMCID: PMC8986479.
60. Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, Belden B, Louiselle D, Nolte N, Biswell R, Pastinen T, Myers A, Schuster J. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(20):1959-61. doi: 10.1056/NEJMc2102051. PubMed PMID: 33755375; PMCID: PMC8008753.
61. Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermudez-Gonzalez MC, Bielak DA, Carreno JM, Chernet RL, Eaker LQ, Ferreri ED, Floda DL, Gleason CR, Hamburger JZ, Jiang K, Kleiner G, Jurczyszak D, Matthews JC, Mendez WA, Nabeel I, Mulder LCF, Raskin AJ, Russo KT, Salimbangon AT, Saksena M, Shin AS, Singh G, Sominsky LA, Stadlbauer D, Wajnberg A, Simon V. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372-4. doi: 10.1056/NEJMc2101667. PubMed PMID: 33691060; PMCID: PMC8008743.
62. Tauzin A, Gong SY, Beaudoin-Bussieres G, Vezina D, Gasser R, Nault L, Marchitto L, Benlarbi M, Chatterjee D, Nayrac M, Laumaea A, Prevost J, Boutin M, Sannier G, Nicolas A, Bourassa C, Gendron-Lepage G, Medjahed H, Goyette G, Bo Y, Perreault J, Gokool L, Morrisseau C, Arlotto P, Bazin R, Dube M, De Serres G, Brousseau N, Richard J, Rovito R, Cote M, Tremblay C, Marchetti GC, Duerr R, Martel-Laferriere V, Kaufmann DE, Finzi A. Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022;30(1):97-109 e5. doi: 10.1016/j.chom.2021.12.004. PubMed PMID: 34953513; PMCID: PMC8639412.
63. Epsi NJ, Richard SA, Lindholm DA, Mende K, Ganesan A, Huprikar N, Lalani T, Fries AC, Maves RC, Colombo RE, Larson DT, Smith A, Chi SW, Maldonado CJ, Ewers EC, Jones MU, Berjohn CM, Libraty DH, Edwards MS, English C, Rozman JS, Mody RM, Colombo CJ, Samuels EC, Nwachukwu P, Tso MS, Scher AI, Byrne C, Rusiecki J, Simons MP, Tribble D, Broder CC, Agan BK, Burgess TH, Laing ED, Pollett SD, Group EC-CS. Understanding ‘hybrid immunity’: comparison and predictors of humoral immune responses to SARS-CoV-2 infection and COVID-19 vaccines. Clin Infect Dis. 2022. doi: 10.1093/cid/ciac392. PubMed PMID: 35608504; PMCID: PMC9213853.
64. Reynolds CJ, Pade C, Gibbons JM, Butler DK, Otter AD, Menacho K, Fontana M, Smit A, Sackville-West JE, Cutino-Moguel T, Maini MK, Chain B, Noursadeghi M, Network UKCIC, Brooks T, Semper A, Manisty C, Treibel TA, Moon JC, Investigators UKC, Valdes AM, McKnight A, Altmann DM, Boyton R. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science. 2021. doi: 10.1126/science.abh1282. PubMed PMID: 33931567; PMCID: PMC8168614.
65. Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, Cheng S, Sobhani K. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981-4. doi: 10.1038/s41591-021-01325-6. PubMed PMID: 33795870; PMCID: PMC8205849.
66. Mesin L, Schiepers A, Ersching J, Barbulescu A, Cavazzoni CB, Angelini A, Okada T, Kurosaki T, Victora GD. Restricted Clonality and Limited Germinal Center Reentry Characterize Memory B Cell Reactivation by Boosting. Cell. 2020;180(1):92-106 e11. doi: 10.1016/j.cell.2019.11.032. PubMed PMID: 31866068; PMCID: PMC6958527.
67. Sanyal M, Holmes TH, Maecker HT, Albrecht RA, Dekker CL, He XS, Greenberg HB. Diminished B-Cell Response After Repeat Influenza Vaccination. J Infect Dis. 2019;219(10):1586-95. doi: 10.1093/infdis/jiy685. PubMed PMID: 30496437; PMCID: PMC6473172.
68. Hansen L, Zhou F, Amdam H, Trieu MC, Cox RJ. Repeated Influenza Vaccination Boosts and Maintains H1N1pdm09 Neuraminidase Antibody Titers. Front Immunol. 2021;12:748264. doi: 10.3389/fimmu.2021.748264. PubMed PMID: 34721417; PMCID: PMC8551669.
69. Hammerman A, Sergienko R, Friger M, Beckenstein T, Peretz A, Netzer D, Yaron S, Arbel R. Effectiveness of the BNT162b2 Vaccine after Recovery from Covid-19. N Engl J Med. 2022;386(13):1221-9. doi: 10.1056/NEJMoa2119497. PubMed PMID: 35172072; PMCID: PMC8908846.
70. Parry H, Bruton R, Stephens C, Bentley C, Brown K, Amirthalingam G, Hallis B, Otter A, Zuo J, Moss P. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines. 2022;7(1):14. doi: 10.1038/s41541-022-00432-w. PubMed PMID: 35087066; PMCID: PMC8795435.
71. Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, Meardon N, Faustini S, Al-Taei S, Moore SC, Tipton T, Hering LM, Angyal A, Brown R, Nicols AR, Gillson N, Dobson SL, Amini A, Supasa P, Cross A, Bridges-Webb A, Reyes LS, Linder A, Sandhar G, Kilby JA, Tyerman JK, Altmann T, Hornsby H, Whitham R, Phillips E, Malone T, Hargreaves A, Shields A, Saei A, Foulkes S, Stafford L, Johnson S, Wootton DG, Conlon CP, Jeffery K, Matthews PC, Frater J, Deeks AS, Pollard AJ, Brown A, Rowland-Jones SL, Mongkolsapaya J, Barnes E, Hopkins S, Hall V, Dold C, Duncan CJA, Richter A, Carroll M, Screaton G, de Silva TI, Turtle L, Klenerman P, Dunachie S, Consortium P. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184(23):5699-714 e11. doi: 10.1016/j.cell.2021.10.011. PubMed PMID: 34735795; PMCID: PMC8519781.
72. Chatterjee D, Tauzin A, Marchitto L, Gong SY, Boutin M, Bourassa C, Beaudoin-Bussieres G, Bo Y, Ding S, Laumaea A, Vezina D, Perreault J, Gokool L, Morrisseau C, Arlotto P, Fournier E, Guilbault A, Delisle B, Levade I, Goyette G, Gendron-Lepage G, Medjahed H, De Serres G, Tremblay C, Martel-Laferriere V, Kaufmann DE, Bazin R, Prevost J, Moreira S, Richard J, Cote M, Finzi A. SARS-CoV-2 Omicron Spike recognition by plasma from individuals receiving BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Rep. 2022;38(9):110429. doi: 10.1016/j.celrep.2022.110429. PubMed PMID: 35216664; PMCID: PMC8823958.
73. Amirthalingam G, Bernal JL, Andrews NJ, Whitaker H, Gower C, Stowe J, Tessier E, Subbarao S, Ireland G, Baawuah F, Linley E, Warrener L, O’Brien M, Whillock C, Moss P, Ladhani SN, Brown KE, Ramsay ME. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat Commun. 2021;12(1):7217. doi: 10.1038/s41467-021-27410-5. PubMed PMID: 34893611; PMCID: PMC8664823.
74. Hall VG, Ferreira VH, Wood H, Ierullo M, Majchrzak-Kita B, Manguiat K, Robinson A, Kulasingam V, Humar A, Kumar D. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol. 2022;23(3):380-5. doi: 10.1038/s41590-021-01126-6. PubMed PMID: 35115679.
75. Grunau B, Goldfarb DM, Asamoah-Boaheng M, Golding L, Kirkham TL, Demers PA, Lavoie PM. Immunogenicity of Extended mRNA SARS-CoV-2 Vaccine Dosing Intervals. JAMA. 2022;327(3):279-81. doi: 10.1001/jama.2021.21921. PubMed PMID: 34860253; PMCID: PMC8642809.
76. Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, Wellington E, Khawam J, Munro K, Cole M, Tranquillini C, Taylor-Kerr A, Hettiarachchi N, Calbraith D, Sajedi N, Milligan I, Themistocleous Y, Corrigan D, Cromey L, Price L, Stewart S, de Lacy E, Norman C, Linley E, Otter AD, Semper A, Hewson J, D’Arcangelo S, Chand M, Brown CS, Brooks T, Islam J, Charlett A, Hopkins S, Group SS. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N Engl J Med. 2022;386(13):1207-20. doi: 10.1056/NEJMoa2118691. PubMed PMID: 35172051; PMCID: PMC8908850.
77. Mehta P, Paul A, Ahmed S, Cherian S, Panthak A, Benny J, Shenoy P. Effectiveness of delayed second dose of AZD1222 vaccine in patients with autoimmune rheumatic disease. Clin Rheumatol. 2022;41(11):3537-42. doi: 10.1007/s10067-022-06247-3. PubMed PMID: 35760938; PMCID: PMC9244552.
78. Romero-Brufau S, Chopra A, Ryu AJ, Gel E, Raskar R, Kremers W, Anderson KS, Subramanian J, Krishnamurthy B, Singh A, Pasupathy K, Dong Y, O’Horo JC, Wilson WR, Mitchell O, Kingsley TC. Public health impact of delaying second dose of BNT162b2 or mRNA-1273 covid-19 vaccine: simulation agent based modeling study. BMJ. 2021;373:n1087. doi: 10.1136/bmj.n1087. PubMed PMID: 33980718; PMCID: PMC8114182.
79. Wallace M, Moulia D, Blain AE, Ricketts EK, Minhaj FS, Link-Gelles R, Curran KG, Hadler SC, Asif A, Godfrey M, Hall E, Fiore A, Meyer S, Su JR, Weintraub E, Oster ME, Shimabukuro TT, Campos-Outcalt D, Morgan RL, Bell BP, Brooks O, Talbot HK, Lee GM, Daley MF, Oliver SE. The Advisory Committee on Immunization Practices’ Recommendation for Use of Moderna COVID-19 Vaccine in Adults Aged >/=18 Years and Considerations for Extended Intervals for Administration of Primary Series Doses of mRNA COVID-19 Vaccines - United States, February 2022. MMWR Morb Mortal Wkly Rep. 2022;71(11):416-21. doi: 10.15585/mmwr.mm7111a4. PubMed PMID: 35298454; PMCID: PMC8942305.
80. Moghadas SM, Vilches TN, Zhang K, Nourbakhsh S, Sah P, Fitzpatrick MC, Galvani AP. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021;19(4):e3001211. doi: 10.1371/journal.pbio.3001211. PubMed PMID: 33882066; PMCID: PMC8092656.
81. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA, 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH, m RNASG. An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med. 2020;383(20):1920-31. doi: 10.1056/NEJMoa2022483. PubMed PMID: 32663912; PMCID: PMC7377258.
82. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, Brachtendorf S, Lorks V, Sikorski J, Hilker R, Becker D, Eller AK, Grutzner J, Boesler C, Rosenbaum C, Kuhnle MC, Luxemburger U, Kemmer-Bruck A, Langer D, Bexon M, Bolte S, Kariko K, Palanche T, Fischer B, Schultz A, Shi PY, Fontes-Garfias C, Perez JL, Swanson KA, Loschko J, Scully IL, Cutler M, Kalina W, Kyratsous CA, Cooper D, Dormitzer PR, Jansen KU, Tureci O. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-9. doi: 10.1038/s41586-020-2814-7. PubMed PMID: 32998157.
83. Cavanaugh AM, Spicer KB, Thoroughman D, Glick C, Winter K. Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination - Kentucky, May-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1081-3. doi: 10.15585/mmwr.mm7032e1. PubMed PMID: 34383732; PMCID: PMC8360277.
84. Bozio CH, Grannis SJ, Naleway AL, Ong TC, Butterfield KA, DeSilva MB, Natarajan K, Yang DH, Rao S, Klein NP, Irving SA, Dixon BE, Dascomb K, Liao IC, Reynolds S, McEvoy C, Han J, Reese SE, Lewis N, Fadel WF, Grisel N, Murthy K, Ferdinands J, Kharbanda AB, Mitchell PK, Goddard K, Embi PJ, Arndorfer J, Raiyani C, Patel P, Rowley EA, Fireman B, Valvi NR, Griggs EP, Levy ME, Zerbo O, Porter RM, Birch RJ, Blanton L, Ball SW, Steffens A, Olson N, Williams J, Dickerson M, McMorrow M, Schrag SJ, Verani JR, Fry AM, Azziz-Baumgartner E, Barron M, Gaglani M, Thompson MG, Stenehjem E. Laboratory-Confirmed COVID-19 Among Adults Hospitalized with COVID-19-Like Illness with Infection-Induced or mRNA Vaccine-Induced SARS-CoV-2 Immunity - Nine States, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1539-44. doi: 10.15585/mmwr.mm7044e1. PubMed PMID: 34735425; PMCID: PMC8568091.
85. Leon TM, Dorabawila V, Nelson L, Lutterloh E, Bauer UE, Backenson B, Bassett MT, Henry H, Bregman B, Midgley CM, Myers JF, Plumb ID, Reese HE, Zhao R, Briggs-Hagen M, Hoefer D, Watt JP, Silk BJ, Jain S, Rosenberg ES. COVID-19 Cases and Hospitalizations by COVID-19 Vaccination Status and Previous COVID-19 Diagnosis - California and New York, May-November 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):125-31. doi: 10.15585/mmwr.mm7104e1. PubMed PMID: 35085222; PMCID: PMC9351527.
86. Grant R, Charmet T, Schaeffer L, Galmiche S, Madec Y, Von Platen C, Cheny O, Omar F, David C, Rogoff A, Paireau J, Cauchemez S, Carrat F, Septfons A, Levy-Bruhl D, Mailles A, Fontanet A. Impact of SARS-CoV-2 Delta variant on incubation, transmission settings and vaccine effectiveness: Results from a nationwide case-control study in France. Lancet Reg Health Eur. 2022;13:100278. doi: 10.1016/j.lanepe.2021.100278. PubMed PMID: 34849500; PMCID: PMC8616730.
87. Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman LS, Ash N, Alroy-Preis S, Huppert A, Milo R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N Engl J Med. 2022;386(23):2201-12. doi: 10.1056/NEJMoa2118946. PubMed PMID: 35613036; PMCID: PMC9165562.
88. Gazit S, Shlezinger R, Perez G, Lotan R, Peretz A, Ben-Tov A, Herzel E, Alapi H, Cohen D, Muhsen K, Chodick G, Patalon T. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Naturally Acquired Immunity versus Vaccine-induced Immunity, Reinfections versus Breakthrough Infections: A Retrospective Cohort Study. Clin Infect Dis. 2022;75(1):e545-e51. doi: 10.1093/cid/ciac262. PubMed PMID: 35380632; PMCID: PMC9047157.
89. Shenai MB, Rahme R, Noorchashm H. Equivalency of Protection From Natural Immunity in COVID-19 Recovered Versus Fully Vaccinated Persons: A Systematic Review and Pooled Analysis. Cureus. 2021;13(10):e19102. doi: 10.7759/cureus.19102. PubMed PMID: 34868754; PMCID: PMC8627252.
90. Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hagglof T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O’Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639-44. doi: 10.1038/s41586-021-03207-w. PubMed PMID: 33461210; PMCID: PMC8221082.
91. Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Viant C, Gaebler C, Cipolla M, Hoffmann HH, Oliveira TY, Oren DA, Ramos V, Nogueira L, Michailidis E, Robbiani DF, Gazumyan A, Rice CM, Hatziioannou T, Bieniasz PD, Caskey M, Nussenzweig MC. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13(577). doi: 10.1126/scitranslmed.abf1555. PubMed PMID: 33288661; PMCID: PMC7857415.
92. Sterlin D, Mathian A, Miyara M, Mohr A, Anna F, Claer L, Quentric P, Fadlallah J, Devilliers H, Ghillani P, Gunn C, Hockett R, Mudumba S, Guihot A, Luyt CE, Mayaux J, Beurton A, Fourati S, Bruel T, Schwartz O, Lacorte JM, Yssel H, Parizot C, Dorgham K, Charneau P, Amoura Z, Gorochov G. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci Transl Med. 2021;13(577). doi: 10.1126/scitranslmed.abd2223. PubMed PMID: 33288662; PMCID: PMC7857408.
93. Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, Al-Khatib HA, Smatti MK, Coyle P, Al-Kanaani Z, Al-Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul-Rahim HF, Nasrallah GK, Al-Kuwari MG, Butt AA, Al-Romaihi HE, Al-Thani MH, Al-Khal A, Bertollini R, Abu-Raddad LJ. Effects of Previous Infection and Vaccination on Symptomatic Omicron Infections. N Engl J Med. 2022;387(1):21-34. doi: 10.1056/NEJMoa2203965. PubMed PMID: 35704396; PMCID: PMC9258753.
94. Altarawneh HN, Chemaitelly H, Hasan MR, Ayoub HH, Qassim S, AlMukdad S, Coyle P, Yassine HM, Al-Khatib HA, Benslimane FM, Al-Kanaani Z, Al-Kuwari E, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Abdul-Rahim HF, Nasrallah GK, Al-Kuwari MG, Butt AA, Al-Romaihi HE, Al-Thani MH, Al-Khal A, Bertollini R, Tang P, Abu-Raddad LJ. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N Engl J Med. 2022;386(13):1288-90. doi: 10.1056/NEJMc2200133. PubMed PMID: 35139269; PMCID: PMC8849180.
95. Andreano E, Paciello I, Piccini G, Manganaro N, Pileri P, Hyseni I, Leonardi M, Pantano E, Abbiento V, Benincasa L, Giglioli G, De Santi C, Fabbiani M, Rancan I, Tumbarello M, Montagnani F, Sala C, Montomoli E, Rappuoli R. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature. 2021;600(7889):530-5. doi: 10.1038/s41586-021-04117-7. PubMed PMID: 34670266; PMCID: PMC8674140.
96. Rodda LB, Morawski PA, Pruner KB, Fahning ML, Howard CA, Franko N, Logue J, Eggenberger J, Stokes C, Golez I, Hale M, Gale M, Jr., Chu HY, Campbell DJ, Pepper M. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell. 2022;185(9):1588-601 e14. doi: 10.1016/j.cell.2022.03.018. PubMed PMID: 35413241; PMCID: PMC8926873.
97. Pape KA, Dileepan T, Kabage AJ, Kozysa D, Batres R, Evert C, Matson M, Lopez S, Krueger PD, Graiziger C, Vaughn BP, Shmidt E, Rhein J, Schacker TW, Khoruts A, Jenkins MK. High-affinity memory B cells induced by SARS-CoV-2 infection produce more plasmablasts and atypical memory B cells than those primed by mRNA vaccines. Cell Rep. 2021;37(2):109823. doi: 10.1016/j.celrep.2021.109823. PubMed PMID: 34610291; PMCID: PMC8463313.
98. Lee JH, Sutton HJ, Cottrell CA, Phung I, Ozorowski G, Sewall LM, Nedellec R, Nakao C, Silva M, Richey ST, Torre JL, Lee WH, Georgeson E, Kubitz M, Hodges S, Mullen TM, Adachi Y, Cirelli KM, Kaur A, Allers C, Fahlberg M, Grasperge BF, Dufour JP, Schiro F, Aye PP, Kalyuzhniy O, Liguori A, Carnathan DG, Silvestri G, Shen X, Montefiori DC, Veazey RS, Ward AB, Hangartner L, Burton DR, Irvine DJ, Schief WR, Crotty S. Long-primed germinal centres with enduring affinity maturation and clonal migration. Nature. 2022;609(7929):998-1004. doi: 10.1038/s41586-022-05216-9. PubMed PMID: 36131022; PMCID: PMC9491273.
99. Zhang Y, Meyer-Hermann M, George LA, Figge MT, Khan M, Goodall M, Young SP, Reynolds A, Falciani F, Waisman A, Notley CA, Ehrenstein MR, Kosco-Vilbois M, Toellner KM. Germinal center B cells govern their own fate via antibody feedback. J Exp Med. 2013;210(3):457-64. doi: 10.1084/jem.20120150. PubMed PMID: 23420879; PMCID: PMC3600904.
100. Schaefer-Babajew D, Wang Z, Muecksch F, Cho A, Loewe M, Cipolla M, Raspe R, Johnson B, Canis M, DaSilva J, Ramos V, Turroja M, Millard KG, Schmidt F, Witte L, Dizon J, Shimelovich I, Yao KH, Oliveira TY, Gazumyan A, Gaebler C, Bieniasz PD, Hatziioannou T, Caskey M, Nussenzweig MC. Antibody feedback regulates immune memory after SARS-CoV-2 mRNA vaccination. Nature. 2022 Dec 6. doi: 10.1038/s41586-022-05609-w. Epub ahead of print. PMID: 36473496.
101. Sutton HJ, Aye R, Idris AH, Vistein R, Nduati E, Kai O, Mwacharo J, Li X, Gao X, Andrews TD, Koutsakos M, Nguyen THO, Nekrasov M, Milburn P, Eltahla A, Berry AA, Kc N, Chakravarty S, Sim BKL, Wheatley AK, Kent SJ, Hoffman SL, Lyke KE, Bejon P, Luciani F, Kedzierska K, Seder RA, Ndungu FM, Cockburn IA. Atypical B cells are part of an alternative lineage of B cells that participates in responses to vaccination and infection in humans. Cell Rep. 2021;34(6):108684. doi: 10.1016/j.celrep.2020.108684. PubMed PMID: 33567273; PMCID: PMC7873835.
102. Bhattacharya D. Instructing durable humoral immunity for COVID-19 and other vaccinable diseases. Immunity. 2022;55(6):945-64. doi: 10.1016/j.immuni.2022.05.004. PubMed PMID: 35637104; PMCID: PMC9085459.
103. Dhenni R, Phan TG. The geography of memory B cell reactivation in vaccine-induced immunity and in autoimmune disease relapses. Immunol Rev. 2020;296(1):62-86. doi: 10.1111/imr.12862. PubMed PMID: 32472583.
104. Buckner CM, Kardava L, El Merhebi O, Narpala SR, Serebryannyy L, Lin BC, Wang W, Zhang X, Lopes de Assis F, Kelly SEM, Teng IT, McCormack GE, Praiss LH, Seamon CA, Rai MA, Kalish H, Kwong PD, Proschan MA, McDermott AB, Fauci AS, Chun TW, Moir S. Interval between prior SARS-CoV-2 infection and booster vaccination impacts magnitude and quality of antibody and B cell responses. Cell. 2022. doi: 10.1016/j.cell.2022.09.032. PubMed PMID: 36257313; PMCID: PMC9513331.
105. Mazzoni A, Vanni A, Spinicci M, Lamacchia G, Kiros ST, Rocca A, Capone M, Di Lauria N, Salvati L, Carnasciali A, Mantengoli E, Farahvachi P, Zammarchi L, Lagi F, Colao MG, Liotta F, Cosmi L, Maggi L, Bartoloni A, Rossolini GM, Annunziato F. SARS-CoV-2 infection and vaccination trigger long-lived B and CD4+ T lymphocytes with implications for booster strategies. J Clin Invest. 2022;132(6). doi: 10.1172/JCI157990. PubMed PMID: 35139036; PMCID: PMC8920339.
106. Kardava L, Sohn H, Youn C, Austin JW, Wang W, Buckner CM, Justement JS, Melson VA, Roth GE, Hand MA, Gittens KR, Kwan RW, Sneller MC, Li Y, Chun TW, Sun PD, Pierce SK, Moir S. IgG3 regulates tissue-like memory B cells in HIV-infected individuals. Nat Immunol. 2018;19(9):1001-12. doi: 10.1038/s41590-018-0180-5. PubMed PMID: 30104633; PMCID: PMC6289069.
107. Schrezenmeier E, Weissenberg SY, Stefanski AL, Szelinski F, Wiedemann A, Lino AC, Dorner T. Postactivated B cells in systemic lupus erythematosus: update on translational aspects and therapeutic considerations. Curr Opin Rheumatol. 2019;31(2):175-84. doi: 10.1097/BOR.0000000000000576. PubMed PMID: 30540579.
108. Tanaka S, Ise W, Baba Y, Kurosaki T. Silencing and activating anergic B cells. Immunol Rev. 2022;307(1):43-52. doi: 10.1111/imr.13053. PubMed PMID: 34908172.
109. Castleman MJ, Stumpf MM, Therrien NR, Smith MJ, Lesteberg KE, Palmer BE, Maloney JP, Janssen WJ, Mould KJ, Beckham JD, Pelanda R, Torres RM. SARS-CoV-2 infection relaxes peripheral B cell tolerance. J Exp Med. 2022;219(6). doi: 10.1084/jem.20212553. PubMed PMID: 35420627; PMCID: PMC9014793.
110. Andrews SF, Chambers MJ, Schramm CA, Plyler J, Raab JE, Kanekiyo M, Gillespie RA, Ransier A, Darko S, Hu J, Chen X, Yassine HM, Boyington JC, Crank MC, Chen GL, Coates E, Mascola JR, Douek DC, Graham BS, Ledgerwood JE, McDermott AB. Activation Dynamics and Immunoglobulin Evolution of Pre-existing and Newly Generated Human Memory B cell Responses to Influenza Hemagglutinin. Immunity. 2019;51(2):398-410 e5. doi: 10.1016/j.immuni.2019.06.024. PubMed PMID: 31350180.
111. Pusnik J, Konig J, Mai K, Richter E, Zorn J, Proksch H, Schulte B, Alter G, Streeck H. Persistent Maintenance of Intermediate Memory B Cells Following SARS-CoV-2 Infection and Vaccination Recall Response. J Virol. 2022;96(15):e0076022. doi: 10.1128/jvi.00760-22. PubMed PMID: 35862718; PMCID: PMC9364791.
112. Kotaki R, Adachi Y, Moriyama S, Onodera T, Fukushi S, Nagakura T, Tonouchi K, Terahara K, Sun L, Takano T, Nishiyama A, Shinkai M, Oba K, Nakamura-Uchiyama F, Shimizu H, Suzuki T, Matsumura T, Isogawa M, Takahashi Y. SARS-CoV-2 Omicron-neutralizing memory B cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci Immunol. 2022;7(70):eabn8590. doi: 10.1126/sciimmunol.abn8590. PubMed PMID: 35113654; PMCID: PMC8939773.
Submitted October 5, 2022 | Accepted October 23, 2022 | Published December 9, 2022
Copyright © 2022 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License