Richard R. Watkins1, Rachael Gowen2, Michail S. Lionakis3, Mahmoud Ghannoum 2, 4
1Department of Medicine, Division of Infectious Diseases, Northeast Ohio Medical University, Rootstown, Ohio
2 Center for Medical Mycology, Department of Dermatology, Case Western Reserve University, Cleveland, Ohio
3 Fungal Pathogenesis Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy & Infectious Diseases, National Institutes of Health, Bethesda, Maryland
4 University Hospitals Cleveland Medical Center, Cleveland, Ohio
Richard R. Watkins, MD, MS, FACP, FIDSA, FISAC
rwatkins@neomed.edu
Watkins RR, Gowen R, Lionakis MS, Ghannoum M. Update on the Pathogenesis, Virulence, and Treatment of Candida auris. Pathogens and Immunity. 2022;7(2): 46-65. doi: 10.20411/pai.v7i2.535.
10.20411/pai.v7i2.535
Candida auris is an emerging, multidrug resistant fungal pathogen that causes considerable morbidity and mortality. First identified in Japan in 2009, it has since been reported in more than 40 countries. C. auris can persist for long periods on different environmental surfaces as well as the skin. Clinical isolates are typically resistant to commonly prescribed antifungal drugs. Increasingly recognized as a cause of infections and outbreaks in nosocomial settings, C. auris is difficult to identify using traditional microbiological methods. One of the main reasons for the ongoing spread of C. auris is the multitude of virulence factors it possesses and uses against its human host that enables fungal persistence on the skin surface. Yet, many of the virulence mechanisms are unknown or remain incompletely understood. In this review, we summarize the evolution of virulence of C. auris, offer recommendations for combating this important human pathogen, and suggest directions for further research.
Candida auris, virulence, antifungal resistance, fungemia, immune responses
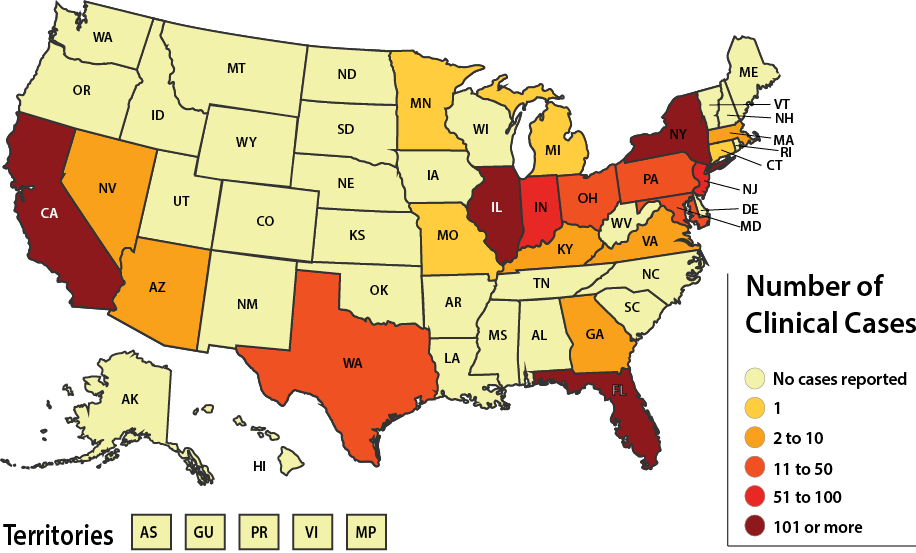
The emergence of Candida auris as a human pathogen has been met with grave concern from clinicians and public health authorities. Since it was first identified in Japan in 2009, C. auris has spread rapidly worldwide [1]. C. auris became a nationally notifiable pathogen in the United States in 2018. The Centers for Disease Control and Prevention (CDC) subsequently designated C. auris an urgent threat due to its resistance to antifungal therapies [2]. As demonstrated in Figure 1, nearly half of all US states reported at least 1 case in 2021. C. auris has evolved a number of novel features that mediate environmental adaptation, host survival, and pathogenicity, which are unique or otherwise similar to other Candida species [3]. Through the use of whole genome sequencing (WGS), 5 different clades based on geography have been recognized: South Asian clade I, East Asian clade II, African clade III, South American clade IV, and clade V from Iran. The clades differ in their antifungal resistance profiles, with clade II exhibiting less resistance than the others, and in their propensity to persist on mammalian skin [4].

Figure 1. Reported clinical cases of Candida auris in the United States, January 01, 2021 to December 31, 2021.
Source: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html#historical
The fungal species most similar to C. auris is C. haemulonii, another phylogenetically related drug-resistant Candida species that is also being increasingly reported from health care facilities [5]. An ascomycetous fungus that grows as yeast, C. auris forms shiny, smooth, whitish colonies on fungal growth media. The name ‘auris’ comes from the Latin word for ear because it was first isolated from the ear canal of a hospitalized patient. The overuse of antifungal agents has been hypothesized as the reason C. auris has become a human pathogen after previously living harmlessly in the environment [6]. Another hypothesis regarding the pathogenicity of C. auris is that it has evolved due to thermal adaptation from climate change [7].
C. auris is often incorrectly identified in the microbiology laboratory when traditional testing methods are employed. It is frequently misidentified as C. haemulonii by the commercial identification system VITEK (bioMérieux, Marcy l’Étoile, France) and as Rhodotorula glutinis by the API-20C AUX (bioMérieux) [8]. These systems employ precast panels of assimilation/growth tests using sets of carbon and nitrogen compounds and are widely used for routine identification of yeast in clinical microbiology laboratories. Many common biochemical tests also misidentify C. auris, most often as R. glutinis or C. haemulonii [9]. Mizusawa et al reported that all C. auris isolates were misidentified as Rhodotorula glutinis by API 20C AUX (bioMérieux); C. haemulonii (except one as C. catenulata) by Becton Dickinson Phoenix (BD Diagnostics, Sparks, MD); C. haemulonii by VITEK 2 (bioMérieux); and C. famata, C. lusitaniae, C. guilliermondii, or C. parapsilosis by MicroScan (Beckman Coulter, Pasadena, CA) [10]. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) is more accurate compared to the other methods for identifying C. auris and allows for subsequent epidemiological characterization of strains [11].
Recently, new formulations of chromogenic media have been specifically developed for identifying C. auris, including CHROMagar Candida Plus (CHROMagar, France) [12] and HiCrome C. auris MDR selective agar (HiMedia, Mumbai, India) [13]. On CHROMagar Candida Plus, C. auris forms characteristic white colonies with blue-green halos that are more evident after 72 hours of incubation at 35°C than after 48 hours. However, distinguishing between closely related species including C. haemulonii, C. pseudohaemulonii, and C. duobushaemulonii requires using additional parameters besides color, including colony size and ability to grow at 35°C. Other identification methods include molecular tests like DNA sequencing and WGS [14]. Future approaches are likely to incorporate machine-learning algorithms with routine microbiological testing [15].
Resistance to antifungal drugs by C. auris is well-documented and is one of the main drivers of its pathogenicity. C. auris exhibits consistently high fluconazole minimal inhibitory concentrations (MICs) and variable susceptibility to the other triazoles, echinocandins, and amphotericin B [16]. Indeed, C. auris is the first Candida species to be classified as multidrug resistant (MDR). Of even greater concern is the emergence of C. auris strains that are pan-resistant, although novel antifungals such as ibrexafungerp and fosmanogepix have demonstrated in vitro and in vivo efficacy against C. auris [17]. Ostrowsky et al described 3 patients with pan-resistant C. auris that developed after receiving antifungal medications, including echinocandins [18]. The isolates were initially susceptible to echinocandins; resistance was detected after treatment, indicating that it emerged during therapy. There was no evidence of transmission of the resistant isolates following the initial infections. Thus, patients on antifungal treatment for C. auris should be closely monitored for clinical improvement. Follow-up cultures are recommended, and repeat susceptibility testing should also be conducted, especially for patients who received echinocandins.
The clinical presentation of C. auris is often non-specific and similar to other types of systemic infections [19]. Most of the reported cases have been associated with invasive infections, such as candidemia and infected devices. The majority of C. auris infections occur in adults, and there is a propensity towards critically ill patients in intensive care units (ICUs). The mortality rate for C. auris candidemia is high and ranges from 30% to 72% [19]. Because C. auris, like other Candida species, can be a colonizer of non-sterile body sites (eg, lungs, urine, and skin), it is important to ascertain the signs and symptoms of infection when C. auris is identified in clinical specimens. In addition to candidemia, C. auris has been reported to cause urinary tract infections (UTIs), otitis, wound infections, skin abscesses (often related to catheters), myocarditis, meningitis, and osteomyelitis [20].
The COVID-19 pandemic has led to a large number of critically ill patients admitted to ICUs. Not surprisingly, outbreaks of SARS-CoV-2 and C. auris co-infections have been reported from a number of facilities [21, 22]. A meta-analysis from studies published during the first 18 months of the pandemic found an increased prevalence of antimicrobial resistance (AMR) in patients with COVID-19 who subsequently developed bacterial and fungal infections, including a high prevalence of MDR C. auris [23]. The majority of hospitalized patients with COVID-19 received at least one antibiotic (often azithromycin), a known risk factor for nosocomial fungal infections. Hospital admission, especially to the ICU, also increases the risk of patients with COVID-19 becoming colonized with C. auris, who can then serve as reservoirs for transmission to other patients and the environment. Using WGS, Yadav et al found that 10% of fomite samples contained C. auris in rooms about 8½ days after C. auris colonized patients were admitted [24]. The high effectiveness of 1% sodium hypochlorite against C. auris was noted, thus providing a potential method for reducing its transmission in nosocomial settings.
While it is possible for any individual to become infected with C. auris regardless of health status, a number of risk factors have been identified (Table 1). Pandya et al found the most common age range for C. auris infections was 61 to 70 years [25]. Skin colonization with C. auris also increases the risk for disseminated infection, particularly candidemia [26, 27]. The skin of many nursing home residents is chronically colonized with C. auris, which likely represents an important reservoir for its ongoing spread [28].
Table 1. Risk Factors for Candida auris Infection
• Urinary catheters
• Central venous catheters
• Malignancy
• Chronic kidney disease
• Neutropenia
• Total parenteral nutrition (TPN)
• Increased hospital LOS
• Mechanical ventilation
• Immune compromise
• Recent (ie, within previous 90 days) or ongoing broad-spectrum antibiotic use
• Surgical procedures
• HIV/AIDS
LOS: length of stay
Virulence factors are defined as genes and other advantages that contribute to the pathogenicity of an organism. These factors can be maintained within the genome on pathogenicity islands; these are areas containing segments of genes that directly confer infectious qualities leading to disease in the host [29]. Often these genetic advantages promote pathogen survival in otherwise inhospitable environments, allowing the organism to spread and multiply. In the case of Candida species, C. albicans is considered a model study organism due to the decades of research analyzing its contribution to systemic and vaginal infections. For example, a well-studied C. albicans virulence factor is PHR1, a gene that when expressed allows the yeast to survive in neutral pH environments such as the bloodstream or tissues [30]. In contrast, the RPH2 gene promotes survival in acidic environments such as the vaginal canal. Other Candida species such as C. dubliniensis maintain homologues to these genes; PHR1 and PHR2 serve similar functions for this organism as well [31]. C. maltosa also has homologues EPD1 and EPD2 that function similarly to PHR1 of C. albicans.
The Candida genus also displays some species that are able to change phenotype and morphology, typically called phenotypic switching [32]. When plated on agar, C. albicans can switch from white colonies into a filamentous form. in vivo, these hyphae protrude and force their way into skin layers, gastrointestinal tract, or other boundaries not otherwise able to be accessed by the yeast [32]. When C. auris was first studied for phenotype switching, it did not produce hyphae or pseudohyphae. Yet under high salt conditions and in biofilm formation, C. auris can produce basic pseudohyphae [33]. It should be noted, however, that C. auris lacks candidalysin (ECE1) and hyphal cell wall protein (HWP1), which are essential for full hyphal growth [34].
Perhaps the largest contributing factor to virulence of Candida lies with secreted aspartyl proteinases (SAPS). These enzymes have been implicated in many functions such as adhesion, biofilm, cell-wall formation, and host tissue degradation [35]. SAPS have also been shown to downregulate the immune complement system and evade the immune system altogether via preventing inflammatory responses [36]. C. albicans employs 10 genes encoding SAPS, of which SAP4, SAP5, and SAP6 are the most important for virulence [37]. In C. auris, hydrolases are the predominant secreted enzyme, comprising 42% of their encoded enzymes. Orthologous genes for 4 SAPS have also been identified within the C. auris genome [38]. In one study, C. albicans SAPS were most active at 25°C, 37°C, and 40°C [39]. In comparison, C. auris SAPS were elevated at 42°C, indicating that the yeast might have increased survivability at higher temperatures than other Candida species.
Beyond SAPS, lipases are another group of virulence-implicated enzymes that can contribute to biofilm formation, damage to host cells, and evasion of the immune system [40]. When lipases were inhibited via knock-down experiments, as seen with a C. parapsilosis experiment, the yeast could not effectively evade immune cells and were increasingly taken up by macrophages as compared to the control yeast strains [41]. In addition, C. albicans and C. parapsilosis mutants lacking lipases were less virulent in a neonatal rat model [42]. C. auris is able to secrete phospholipases as well, though this ability is strain-dependent and only present in up to 37.5% of isolates [43]. In comparison, 64% of C. auris strains can produce proteinases [43]. Furthermore, C. auris phospholipases are largely weaker compared to C. albicans phospholipases, with the exception of the CBS 12770 C. auris strain [43].
Another key virulence factor is the ability to form biofilms. Through transcriptomic analysis, it was found that C. auris upregulates adhesin proteins CSA1, IFF4, PGA26, and PGA52 when forming and maintaining biofilms [44]. As the biofilm matures, ABC transporter proteins such as CDR1, SNQ2, and YHD3 activate, and major facilitator superfamily proteins MDR1 and RDC3 are upregulated [44]. Also, adherence factors allow for cell-cell adhesion within the biofilm. Agglutinin-like sequence (ALS) proteins ALS1 and ALS5 of C. auris are hypothesized to contribute to adherence of biofilm formation in C. auris according to transcriptomic analyses [44]. Interestingly, C. auris retains a much lower amount of ALS and other adhesin genes when compared to C. albicans, which may help explain why C. albicans biofilms are more common and robust [38].
C. auris exhibits unique evasion mechanisms to host immune systems. An in vitro model utilizing human neutrophils co-cultured with C. auris did not inhibit fungal growth: indeed, C. auris grew past the initial inoculum amount [45]. Compared to C. albicans, when the experiment was repeated, neutrophils decreased fungal growth by 75%. When C. albicans and C. auris were co-cultured in vitro with human neutrophils, neutrophils primarily targeted and engulfed C. albicans rather than C. auris cells [45]. To further explore this mechanism of action, an in vivo zebrafish model was assessed for C. auris evasion of neutrophils. In this animal model, C. albicans only maintained a 5% survival rate, whereas C. auris appeared much more resilient to neutrophils. When an in vivo sample was cultured, it was found that the host immune system recruited 50% fewer neutrophils for C. auris than C. albicans. Further fluorescent microscopy of the neutrophils from this experiment revealed that neutrophils did not form neutrophil extracellular traps (NETs) when exposed to C. auris in vivo [45]. This indicates that perhaps C. auris has a potent inhibitory effect on neutrophils to avoid innate immune detection, specifically targeting NETS to avoid being engulfed.
To further test host immune response to C. auris bloodstream infection, another study utilized immunocompetent C57BL/6 mice [46]. Mice were infected with C. auris and splenocytes were isolated from surviving mice after 7 days. Flow cytometry was then used to determine levels of immune signaling molecules: programmed cell death protein (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) were measured on the surface of T cells, natural killer cells, macrophages, and natural killer T cells. Results from the flow cytometry revealed PD-1 expression was elevated, especially on macrophages from the spleen. Spearman’s rank correlation coefficient was P = 0.95 when calculated for fungal burden and PD-L1 expressing macrophages. This means that as the growth of systemic C. auris increases, so too does PD-1 signaling. Since PD-1 is considered an inhibitor of T-cell activation, this study provides evidence of a C. auris immune evasion mechanism [47]. Due to these results, there is potential that C. auris interacts with or stimulates PD-1 expression, which will have downstream effects that inhibit T-cell activation and proper immune response [47]. This observation may have therapeutic implications, since antibodies against PD-1 induction would prevent this inhibition of T-cell activation and counteract this immune evasion strategy. However, more research is needed on the exact mechanism that C. auris employs to increase PD-1 expression and evade the immune system.
Before its discovery in 2009, C. auris had been largely misidentified as Candida haemulonii. Using genetic sequencing mechanisms, Vallabhaneni et al distinguished this emerging yeast from other Candidal species such as C. haemulonii [48]. While much of the entire C. auris genome remains unknown, there have been several studies to determine phylogenetic relationships, clade categorization, and virulence factors [49] [50].
Using Illumina and Nanopore sequencing, 7 chromosomes have been identified belonging to C. auris [51]. The pathogen’s genome also shares more homology with that of C. haemulonii than C. albicans and is most closely linked phylogenetically to C. heveicola [51]. It is thought that chromosomal rearrangement may have contributed greatly to the emergence of the 5 C. auris clades: clade I and clade III have translocated DNA from prominent isolates B8441 and B11221, respectively [34]. Despite their genetic divergences, the 4 main clades of C. auris share 98.7% genetic similarity.
Much of the C. auris genome has been uncovered via genetic homology to other candidal organisms and while there are several similarities, C. auris also diverges from common reference species such as C. albicans. For example, through genetic comparison, highly expressed C. albicans virulence genes ECE1 (candidalysin) and HWP1 (hyphal cell wall protein) were found not to be conserved in the C. auris genome [33]. In C. albicans, the HWP1 gene is implicated in hyphal development, a feature which improves survival under high-salinity conditions [34]. It is currently unknown why C. auris does not have these survival genes maintained in its genome. However, the C. auris genome does contain several lytic enzymes to improve pathogenesis, such as hydrolases, transferases, and oxidoreductases, though the expression of these enzymes is often strain-dependent [38]. It should be noted that this enzymatic expression was found to be tantamount to other Candida species such as C. albicans and C. dubliniensis [34].
C. auris may however have an advantage over C. albicans in terms of iron acquisition and temperature tolerance. Most C. auris strains have active hemolysin enzymes that help competitively sequester iron for faster growth and increased spread [52]. C. auris also responds more aggressively to temperature stress than C. albicans: when compared at 42°C, C. auris secreted higher levels of protective aspartyl proteinase compared to C. albicans [39]. Currently, however, the whole C. auris genome has not been fully mapped; many protein sequences have been documented on public databases such as NCBI, but their functions remain unknown.
As previously mentioned, C. auris displays a formidable ability to generate biofilms, wherein the yeast secretes mannan-glucan polysaccharides to form a barrier to the external environment [50]. C. auris cells anchor themselves to a surface via adhesin proteins to initiate a biofilm state. Once tethered, the layers of biofilm thicken to form an impenetrable barrier from the immune system and antifungal therapeutics. Studies have also shown that genes controlling efflux pumps are upregulated during biofilm formation, perhaps aiding in ejecting antifungal agents [50]. When compared to C. albicans, C. auris has a decreased propensity for biofilm formation and adherence (P = 0.01) [43].
However, specific C. auris strains may show a higher propensity for biofilm generation, with clade III more commonly forming aggregates leading to biofilm formation [50]. This clade maintains a higher expression of adhesins and upregulation of adhesin genes to achieve this protective barrier. The age of biofilms also contributes to antifungal resistance, since mature biofilms have shown more pan-resistance to all 3 classes (azoles, echinocandins, and polyenes) of antifungals [53]. Inhibition of biofilm efflux pumps restored susceptibility to fluconazole in one study, exemplifying the ability of mannan and glucan to otherwise sequester azole antifungals [53].
Biofilms have also proven to be a contributing factor to nosocomial spread in multiple case studies. Indeed, health care professionals are largely implicated as vectors for C. auris via provider-patient contact through skin colonization or surface contamination [54]. Candida species were found on 36.4% of hospital floors surveyed, 23.5% of sink drains, and 9.1% of all high-touch surfaces [54]. In another study, C. auris was allowed to grow biofilms and was tested against 13 commonly used hospital disinfectants. Overall, 50% of the products failed to prevent cell viability, 58% did not prevent transfer of C. auris, and 75% of the disinfectants could not prevent biofilm regrowth [55].
Biofilms therefore pose a large threat to proper sanitation in hospital settings. In a study on the effectiveness of 9 disinfectants against several Candida species, common surface cleaning chemicals such as Purell, Lysol, and Virex had little-to-no effect on C. albicans, C. glabrata, and C. auris growth [56]. Only bleach agents such as Clorox, OxyCide, and Oxivir TB reduced colony formation significantly [56]. Unfortunately, ammonium-based cleaning agents are widely used across hospitals, even though they have proven less effective than bleach [56]. It has thus been proposed that alternative strategies may be required or used in tandem with cleaning agents. In lieu of this dilemma, photodynamic therapy has been studied to avoid antifungal resistance and the additional protection offered by biofilms. Certain wavelengths of light may be able to bypass the protective biofilm layer and penetrate pathogenic cells, if used in tandem with photosensitizing compounds and molecular oxygen [57]. The ability of light to target multiple cellular components also decreases the chance of developing resistance mechanisms [57].
In one such study investigating the ability of UV light to penetrate C. auris colonization, a decontamination device emitting 254 nm of UV-C light was assessed for germicidal properties. Notably, a 10-minute exposure time did not reduce C. auris growth as much as C. glabrata and C. albicans, further attesting to the robust nature of this pathogenic organism [58]. An exposure time of 20 to 30 minutes was required to significantly reduce growth of C. auris [56]. Additionally, the diameter of the spread of C. auris contributed to colony formation under UV light. C. auris was tested for colony reduction at spreads of 10 mm, 20 mm, and 40 mm diameter. When exposed to UV light at a distance of 5 feet, colonies with a larger surface area of 40 mm were more effectively reduced than at 10 mm [58]. Thus, it was hypothesized that the larger cell size of C. auris in comparison to bacteria and the surface area of the pathogen may contribute to the longer exposure time, as it may take longer for the UV light to properly penetrate cells underneath a protective biofilm layer [58]. Photodynamic therapy therefore represents a promising alternative to clinical antifungals that may overcome resistance mechanisms due to biofilm.
C. auris uniquely colonizes host skin externally as well as systemically, causing infections that can enter the bloodstream and spread throughout the body. This invasive form of candidiasis is recognized as candidemia and can often prove more fatal than cutaneous infections [59]. Fortunately, mucocutaneous infections of Candida species are typically more common, though it should be recognized that vulnerable immunocompromised and otherwise sick individuals in nosocomial settings are most susceptible and at risk [59]. This fact alone creates urgency for better antimicrobial cleaning agents in hospitals to avoid nosocomial spread among patients, as well as antifungals that are effective against systemic infections caused by C. auris.
There are currently several animal models for evaluating C. auris antifungal susceptibility, mainly utilizing small mammalian species such as mouse or guinea pig. One such process involves in vivo cutaneous infection of guinea pig or mouse, in which the animal hair is shaved to better evaluate skin infection of the yeast [60]. Skin samples from sacrificed animals can then be assessed for colony formation through plating on nutrient-rich media. Colonies are then counted to evaluate growth and cutaneous spread is visually assessed on the shaved animal [60]. This model is often utilized to determine the pathogenicity of various C. auris strains, as well as host response to skin colonization and therapeutic approaches to decolonize the skin.
This model can also be utilized to test novel antifungal agents against cutaneous infection. The most effective strategy for evaluating new antifungal agents is through susceptibility testing. This process involves initially evaluating the MIC. Standard MIC values for a candidal organism can be found on the ARTEMIS Global Antifungal Surveillance Program database, which holds information for about 5,346 Candida isolates [61]. This compiled information gives a good baseline for susceptibility testing for both the species and type of drug administered. Though MIC values are technically arbitrary, they give a good sense of comparison especially when testing novel antifungals. Susceptibility testing can be achieved by making serial dilutions of the drug and exposing the organism of interest to varying concentrations [60]. A positive finding of the in vitro testing provides rationale for moving candidate compounds forward for in vivo evaluation, as well as provides dosage guidance.
Animal models are also useful for measuring and assessing systemic host response to the pathogen. In one such study, skin colonization of C. auris was found to reside in deeper tissue, leading to invasive candidemia [62]. Innate and adaptive immune responses were then evaluated as a model for host defense. Both CD4+ and CD8+ T cells were found on the skin surface in large quantities, with helper CD4+ Th17 cells accumulating in deeper skin layers [62]. Studies such as these were crucial to discovering the prominent role of IL-17 in cutaneous and subcutaneous C. auris infection. Indeed, IL-17 deficient mice do not mount as protective a response against cutaneous C. auris, indicating that IL-17 is crucial for immune detection and response to this fungal pathogen [62].
Mouse models can also provide an estimate of virulence for C. auris when compared to other Candida species. Invasive C. albicans, for example, has been well-documented in scientific literature for decades, and thus provides a good baseline model for novel fungal pathogens. In one such study, C. auris was compared with C. haemulonii, C. glabrata, and C. albicans in vivo [63]. One (1) × 105 colony forming units (CFUs)/mouse inoculum was injected into the tail vein of healthy female mice that were then monitored for up to 30 days. After sacrificing, mouse kidney, liver, spleen, and lung were aseptically harvested and assessed for fungal burden. Notably, the median survival time (MST) of C. auri- infected mice was 16 to 17 days, with roughly a 30%-40% survival rate. This was comparable to C. albicans MST of 13 days and survival rate of 20%. For reference, C. glabrata obtained an MST of 19 days with 30% survival, while C. haemulonii had 100% survival.
Beyond murine models, some researchers have opted for fly models to test virulence and antifungal treatments. In 2019, one research group posited that the species Drosophila melanogaster could be used as a model of C. auris infection [46]. Previous D. melanogaster models had shown promise with Aspergillus fungal strains [64]. Flies used in this study were Toll deficient, providing a streamlined, immune deficient response to infection. Flies could also be bred and ready for infection in a mere 3 weeks, creating a time-efficient animal model for fungal infection.
The 2019 study with C. auris then utilized female flies infected with C. auris, using C. albicans as a control [46]. Though in vitro growth of C. auris was comparably slower than C. albicans, C. auris infection proved more fatal, with over 80% of C. auris infected flies dying before the 7-day mark, compared to 67% of C. albicans infected flies. This finding correlates with expected pathogenicity of C. auris, supporting the idea that fly models may be useful for evaluating the virulence as well as the antifungal activity of candidate antifungal compounds. Furthermore, assessment of fungal burden proved simple in this experimental model: flies can be sacrificed through freezing and then directly homogenized in a tube by vortexing. The resulting mixture can then be directly plated on agar and grown for colony counting.
Overall, animal models are vital for understanding this novel pathogen and modeling its route of infection. Utilizing both murine and insect models can give unique insights into host response and provide unique benefits in the form of cost and time efficiency. Ultimately, much is still unknown about C. auris, but maintaining multiple forms of animal models provides the tools necessary to uncover these mysteries.
In cases of fungal infection, 3 main classes of antifungal medication are often administered for clinical treatment: azoles (eg, fluconazole), polyenes (eg, amphotericin B), and echinocandins (eg, caspofungin) [65]. The CDC currently estimates that 90% of C. auris strains maintain resistance to fluconazole, 30% to amphotericin B, and 5% to echinocandins [65]. There are several factors that have been identified to contribute to antifungal resistance of C. auris, including biofilm formation, efflux pumps, genetic predisposition, and cladal phylogeny. Often multiple factors cooperate in tandem to increase overall resistance for the organism.
First in the arsenal of this pathogenic yeast are efflux pumps, which are transport channels within the cell membrane that eject molecules that may threaten survival. Of note, C. auris possesses 2 major efflux pumps implicated in antifungal resistance: the ATP Binding Cassette (ABC) and Major Facilitator Superfamiliy (MFS) transporters [65]. These pumps are most often implicated in azole resistance and were first identified due to their homology to C. albicans efflux pump genes [65]. The C. auris gene CDR1 was identified as the ortholog for ABC transporters, and proof-of-function was determined via gene knockout, wherein 2 independent studies found that CDR1 removal increased azole susceptibility of the yeast [66].
A second gene of interest in terms of antifungal resistance is ERG11, an ergosterol gene crucial to C. auris cell membrane development. ERG11 specifically encodes the enzyme lanosterol 14-alpha-demethylase, which converts lanosterol into ergosterol for cell membrane structure and integrity [67]. Mutations to ERG11 are correlated with increased azole resistance, with 3 common “hot spot” regions in the gene that are most often mutated. C. auris strains with changes to Y132F or K143R are the most implicated as responsible for the increased resistance. Studies analyzing this mutation indicated that strains with Y132F or K143R substitutions caused resistance to double in C. auris strains when compared to a Saccharomyces cerevisiae control [68].
Another gene of interest in C. auris antifungal resistance is FKS, wherein FKS1 and FKS2 encode 2 subunits of a β (1,3) D-glucan synthase [69]. This enzyme is crucial for the biosynthesized glucan components of the fungal cell wall. Echinocandin antifungals target this enzyme to prevent cell wall regeneration and formation, effectively creating holes in the membrane leading to fungal mortality. Furthermore, prevention of new glucan deposition in the fungal membrane inhibits growth of the pathogen. Mutations to the FKS genes, specifically an S639F amino acid substitution, have been shown to drastically increase overall echinocandin resistance [65].
Beyond mutations, C. auris also employs a common bacterial and fungal strategy for survival: the formation of biofilms. Within a standard biofilm, there is a layer of fixed sessile cells that tightly adhere to one another, and a separate layer of planktonic surface cells capable of breaking free from the biofilm, allowing the spread of infection [53]. C. auris is capable of growing both cell types, with planktonic cells living freely in suspension and adherent cells forming a biofilm, and thus antifungals may have a hard time working effectively against both forms. There is also the added issue of biofilm secretions, a mixture of glucan and mannan polysaccharides, protein, and DNA that form a protective barrier from antifungals [70]. In this way, biofilms act as a beneficial community providing both a shield and a genetically varied ecosystem as protection for the organism.
C. auris strains growing as biofilms can also maintain several unique characteristics, owing to the variation of genetic material within the community. For example, cells within the biofilm with upregulated efflux pump genes will help shield other cells from azole penetration. Studies have indicated that C. auris living in biofilms exhibit increased function of ABC and MFS transporters, which increases the overall antifungal resistance of the cellular community [53]. Additionally, it can be inferred that any cells containing the necessary FKS or ERG11 mutations would also provide protection against other classes of antifungals. Therefore, biofilms provide an extra layer of defense in combination with other mutations that may confer resistance.
Unfortunately, biofilms also contribute to nosocomial infection. With the physical protective layer of polysaccharides provided by the collective biofilm, colonies may be able to survive in environments such as hospital beds, catheters, hospital trays, sinks, and the surface of human skin [53]. This may afford cells protection from any conditions of fomites that may otherwise be inhospitable, such as temperature, material, pH, chemical treatments, etc. This circumstance is especially concerning as immune compromised or otherwise sick individuals within hospital settings may be even more susceptible to C. auris infection. Particularly, immunosuppressed individuals may be more prone to severe candidemia and thus the infection would increase fatality compared to infections in an immunocompetent patient.
There is also a correlation between C. auris clade and antifungal resistance mechanisms. As mentioned previously, certain clades exhibit different levels of antifungal susceptibility. For example, research on the South African clade (clade III) demonstrates high azole resistance, but lower polyene and echinocandin resistance [71]. In this particular study, 12 out of 13 clade I isolates were resistant to both fluconazole and amphotericin B. All 13 of these samples also maintained ERG11 mutations at the Y123F site [71], though it should be noted that 13 isolates are a small sample size to generalize overall resistance of the clade as 100%.
With all of these contributing factors strengthening antifungal resistance, researchers have had to employ new strategies to fight this tenacious pathogen. One such technique involves multidrug treatments or combination therapy, wherein synergistic effects from antifungals add the extra coverage needed to kill resistant isolates. For example, Gharehbolagh et al noted synergism between voriconazole and micafungin (100%), flucytosine and amphotericin B (7%), and flucytosine with micafungin (7%) [72]. However, no synergistic effects were noted with any other combination of traditional antifungals. Furthermore, this study evaluated antifungals in combination with antibiotics. Of these experiments, only caspofungin and colistin showed prominent synergism (100%), while fluconazole with miltefosine, ivermectin, or nafcillin failed to show any synergistic effects. This calls for more studies to identify combinations that have potent C. auris inhibitory activity.
One last technique is currently under evaluation as an effective antifungal treatment: the combination of a novel drug with photodynamic therapy. As previously mentioned, photodynamic therapy may provide a way to inhibit biofilms without the added concern of developing antifungal resistance. This novel form of treatment has been researched in tandem with a novel antifungal agent called ibrexafungerp, which has shown the ability to overcome resistant strains of C. auris, as well as other Candida species. The mechanism of action of ibrexafungerp targets glucan synthesis of the cell wall, specifically inhibiting β-(1,3)-D-glucan development, much like echinocandins, even though it has subtle differences between it and traditional candins [73]. There is in vitro evidence in these drug trials that ibrexafungerp can act on biofilms, azole-resistant strains, echinocandin-resistant strains, as well as Aspergillus and non-mold, pathogenic fungi [74]. This pharmaceutical looks very promising for treating clinical candidiasis, and ibrexafungerp was recently approved by the FDA for the treatment of vulvovaginal candidiasis and will continue to be evaluated for other diseases [73].
The emergence of C. auris as a human pathogen is a serious threat to public health. Since C. auris was first identified, a considerable amount of scientific investigation has led to important discoveries regarding its pathogenesis and virulence mechanisms. However, further research is necessary to understand the global epidemiology of multidrug-resistant C. auris infections and to elucidate additional risk factors, modes of transmission, novel therapeutic options, and effective environmental cleaning and disinfection methods against C. auris in order to improve patient outcomes.
The authors report no financial support for the research, authorship, and/or publication related to this article. This work was supported in part by the Division of Intramural Research of the National Institute of Allergy & Infectious Diseases, National Institutes of Health (to MSL; ZIA AI001175) and (AI145289 to MG).
Dr. Mahmoud Ghannoum serves as a Senior Editor and Dr. Richard Watkins serves as an Associate Editor for Pathogens and Immunity. Dr. Watkins serves as a paid consultant to BioMe´rieux and has received research funding from Allergan. The authors report no other competing financial interests.
1. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41-4. doi: 10.1111/j.1348-0421.2008.00083.x. PubMed PMID: 19161556.
2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. doi: 10.15620/cdc:82532. Available from: https://stacks.cdc.gov/view/cdc/82532.
3. Allert S, Schulz D, Kammer P, Grossmann P, Wolf T, Schauble S, Panagiotou G, Brunke S, Hube B. From environmental adaptation to host survival: Attributes that mediate pathogenicity of Candida auris. Virulence. 2022;13(1):191-214. doi: 10.1080/21505594.2022.2026037. PubMed PMID: 35142597; PMCID: PMC8837256.
4. Chow NA, Munoz JF, Gade L, Berkow EL, Li X, Welsh RM, Forsberg K, Lockhart SR, Adam R, Alanio A, Alastruey-Izquierdo A, Althawadi S, Arauz AB, Ben-Ami R, Bharat A, Calvo B, Desnos-Ollivier M, Escandon P, Gardam D, Gunturu R, Heath CH, Kurzai O, Martin R, Litvintseva AP, Cuomo CA. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio. 2020;11(2):e03364-19. doi: 10.1128/mBio.03364-19. PubMed PMID: 32345637; PMCID: PMC7188998.
5. Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, Maor Y, Tarabia J, Schechner V, Adler A, Finn T. Multidrug-Resistant Candida haemulonii and C. auris, Tel Aviv, Israel. Emerg Infect Dis. 2017;23(1):195-203. doi: 10.3201/eid2302.161486. PubMed PMID: 28098529; PMCID: PMC5324804.
6. Mohsin J, Weerakoon S, Ahmed S, Puts Y, Al Balushi Z, Meis JF, Al-Hatmi AMS. A Cluster of Candida auris Blood Stream Infections in a Tertiary Care Hospital in Oman from 2016 to 2019. Antibiotics (Basel). 2020;9(10):638. doi: 10.3390/antibiotics9100638. PubMed PMID: 32987692; PMCID: PMC7598619.
7. Casadevall A, Kontoyiannis DP, Robert V. Environmental Candida auris and the Global Warming Emergence Hypothesis. mBio. 2021;12(2):e00360-21. doi: 10.1128/mBio.00360-21. PubMed PMID: 33727350; PMCID: PMC8092241.
8. Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang HC. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol. 2011;49(9):3139-42. doi: 10.1128/JCM.00319-11. PubMed PMID: 21715586; PMCID: PMC3165631.
9. Chowdhary A, Sharma C, Meis JF. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017;13(5):e1006290. doi: 10.1371/journal.ppat.1006290. PubMed PMID: 28542486; PMCID: PMC5436850.
10. Mizusawa M, Miller H, Green R, Lee R, Durante M, Perkins R, Hewitt C, Simner PJ, Carroll KC, Hayden RT, Zhang SX. Can Multidrug-Resistant Candida auris Be Reliably Identified in Clinical Microbiology Laboratories? J Clin Microbiol. 2017;55(2):638-40. doi: 10.1128/JCM.02202-16. PubMed PMID: 27881617; PMCID: PMC5277535.
11. Girard V, Mailler S, Chetry M, Vidal C, Durand G, van Belkum A, Colombo AL, Hagen F, Meis JF, Chowdhary A. Identification and typing of the emerging pathogen Candida auris by matrix-assisted laser desorption ionisation time of flight mass spectrometry. Mycoses. 2016;59(8):535-8. doi: 10.1111/myc.12519. PubMed PMID: 27292939.
12. Tamura T, Alshahni MM, Makimura K. Evaluation of CHROMagar Candida Plus chromogenic agar for the presumptive identification of Candida auris. Microbiol Immunol. 2022;66(6):292-8. doi: 10.1111/1348-0421.12973. PubMed PMID: 35229341.
13. Das S, Singh S, Tawde Y, Chakrabarti A, Rudramurthy SM, Kaur H, Shankarnarayan SA, Ghosh A. A Selective Medium for Isolation and Detection of Candida auris, an Emerging Pathogen. J Clin Microbiol. 2021;59(2):e00326-20. doi: 10.1128/JCM.00326-20. PubMed PMID: 33208474; PMCID: PMC8111121.
14. Lockhart SR, Lyman MM, Sexton DJ. Tools for Detecting a “Superbug”: Updates on Candida auris Testing. J Clin Microbiol. 2022;60(5):e0080821. doi: 10.1128/jcm.00808-21. PubMed PMID: 34985980; PMCID: PMC9116168.
15. Pezzotti G, Kobara M, Asai T, Nakaya T, Miyamoto N, Adachi T, Yamamoto T, Kanamura N, Ohgitani E, Marin E, Zhu W, Nishimura I, Mazda O, Nakata T, Makimura K. Raman Imaging of Pathogenic Candida auris: Visualization of Structural Characteristics and Machine-Learning Identification. Front Microbiol. 2021;12:769597. doi: 10.3389/fmicb.2021.769597. PubMed PMID: 34867902; PMCID: PMC8633489.
16. Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, Tarai B, Singh A, Upadhyaya G, Upadhyay S, Yadav P, Singh PK, Khillan V, Sachdeva N, Perlin DS, Meis JF. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018;73(4):891-9. doi: 10.1093/jac/dkx480. PubMed PMID: 29325167.
17. Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. In Vitro and In Vivo Evaluation of the Antifungal Activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother. 2018;62(3):e02319-17. doi: 10.1128/AAC.02319-17. PubMed PMID: 29311065; PMCID: PMC5826120.
18. Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, Group CaIW. Candida auris Isolates Resistant to Three Classes of Antifungal Medications - New York, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(1):6-9. doi: 10.15585/mmwr.mm6901a2. PubMed PMID: 31917780; PMCID: PMC6973342.
19. Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 2018;6:69. doi: 10.1186/s40560-018-0342-4. PubMed PMID: 30397481; PMCID: PMC6206635.
20. Chowdhary A, Voss A, Meis JF. Multidrug-resistant Candida auris: ‘new kid on the block’ in hospital-associated infections? J Hosp Infect. 2016;94(3):209-12. doi: 10.1016/j.jhin.2016.08.004. PubMed PMID: 27634564.
21. Janniger EJ, Kapila R. Public health issues with Candida auris in COVID-19 patients. World Med Health Policy. 2021;13(4):766-72. doi: 10.1002/wmh3.472. PubMed PMID: 34909239; PMCID: PMC8661744.
22. Thoma R, Seneghini M, Seiffert SN, Vuichard Gysin D, Scanferla G, Haller S, Flury D, Boggian K, Kleger GR, Filipovic M, Nolte O, Schlegel M, Kohler P. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob Resist Infect Control. 2022;11(1):12. doi: 10.1186/s13756-022-01052-8. PubMed PMID: 35063032; PMCID: PMC8777447.
23. Kariyawasam RM, Julien DA, Jelinski DC, Larose SL, Rennert-May E, Conly JM, Dingle TC, Chen JZ, Tyrrell GJ, Ronksley PE, Barkema HW. Antimicrobial resistance (AMR) in COVID-19 patients: a systematic review and meta-analysis (November 2019-June 2021). Antimicrob Resist Infect Control. 2022;11(1):45. doi: 10.1186/s13756-022-01085-z. PubMed PMID: 35255988; PMCID: PMC8899460.
24. Yadav A, Singh A, Wang Y, Haren MHv, Singh A, de Groot T, Meis JF, Xu J, Chowdhary A. Colonisation and Transmission Dynamics of Candida auris among Chronic Respiratory Diseases Patients Hospitalised in a Chest Hospital, Delhi, India: A Comparative Analysis of Whole Genome Sequencing and Microsatellite Typing. Journal of Fungi. 2021;7(2):81. PubMed PMID: doi: 10.3390/jof7020081.
25. Pandya N, Cag Y, Pandak N, Pekok AU, Poojary A, Ayoade F, Fasciana T, Giammanco A, Caskurlu H, Rajani DP, Gupta YK, Balkan, II, Khan EA, Erdem H. International Multicentre Study of Candida auris Infections. J Fungi (Basel). 2021;7(10):878. doi: 10.3390/jof7100878. PubMed PMID: 34682299; PMCID: PMC8539607.
26. Ferrer Gomez C, Solis Albamonte P, Delgado Navarro C, Salvador Garcia C, Tormo Palop N, Andres Ibanez JA. Analysis of Candida auris candidemia cases in an Intensive Care Unit of a tertiary hospital. Rev Esp Anestesiol Reanim (Engl Ed). 2021;68(8):431-6. doi: 10.1016/j.redare.2020.10.006. PubMed PMID: 34538766.
27. Garcia-Bustos V, Salavert M, Ruiz-Gaitan AC, Cabanero-Navalon MD, Sigona-Giangreco IA, Peman J. A clinical predictive model of candidaemia by Candida auris in previously colonized critically ill patients. Clin Microbiol Infect. 2020;26(11):1507-13. doi: 10.1016/j.cmi.2020.02.001. PubMed PMID: 32061792.
28. Huang X, Welsh RM, Deming C, Proctor DM, Thomas PJ, Program NCS, Gussin GM, Huang SS, Kong HH, Bentz ML, Vallabhaneni S, Chiller T, Jackson BR, Forsberg K, Conlan S, Litvintseva AP, Segre JA. Skin Metagenomic Sequence Analysis of Early Candida auris Outbreaks in U.S. Nursing Homes. mSphere. 2021;6(4):e0028721. doi: 10.1128/mSphere.00287-21. PubMed PMID: 34346704; PMCID: PMC8386442.
29. Yang YL. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36(4):223-8. PubMed PMID: 14723249.
30. Saporito-Irwin SM, Birse CE, Sypherd PS, Fonzi WA. PHR1, a pH-regulated gene of Candida albicans, is required for morphogenesis. Mol Cell Biol. 1995;15(2):601-13. doi: 10.1128/MCB.15.2.601. PubMed PMID: 7823929; PMCID: PMC231914.
31. Heinz WJ, Kurzai O, Brakhage AA, Fonzi WA, Korting H-C, Frosch M, Mühlschlegel FA. Molecular responses to changes in the environmental pH are conserved between the fungal pathogens Candida dubliniensis and Candida albicans. International Journal of Medical Microbiology. 2000;290(3):231-8. doi: 10.1016/s1438-4221(00)80120-4.
32. Thompson DS, Carlisle PL, Kadosh D. Coevolution of morphology and virulence in Candida species. Eukaryot Cell. 2011;10(9):1173-82. doi: 10.1128/EC.05085-11. PubMed PMID: 21764907; PMCID: PMC3187052.
33. Sherry L, Ramage G, Kean R, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg Infect Dis. 2017;23(2):328-31. doi: 10.3201/eid2302.161320. PubMed PMID: 28098553; PMCID: PMC5324806.
34. Munoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, Farrer RA, Litvintseva AP, Cuomo CA. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. 2018;9(1):5346. doi: 10.1038/s41467-018-07779-6. PubMed PMID: 30559369; PMCID: PMC6297351.
35. de Jong AW, Hagen F. Attack, Defend and Persist: How the Fungal Pathogen Candida auris was Able to Emerge Globally in Healthcare Environments. Mycopathologia. 2019;184(3):353-65. doi: 10.1007/s11046-019-00351-w. PubMed PMID: 31209693.
36. Rapala-Kozik M, Bochenska O, Zajac D, Karkowska-Kuleta J, Gogol M, Zawrotniak M, Kozik A. Extracellular proteinases of Candida species pathogenic yeasts. Mol Oral Microbiol. 2018;33(2):113-24. doi: 10.1111/omi.12206. PubMed PMID: 29139623.
37. Lee SA, Jones J, Hardison S, Kot J, Khalique Z, Bernardo SM, Lazzell A, Monteagudo C, Lopez-Ribot J. Candida albicans VPS4 is required for secretion of aspartyl proteases and in vivo virulence. Mycopathologia. 2009;167(2):55-63. doi: 10.1007/s11046-008-9155-7. PubMed PMID: 18814053; PMCID: PMC5898969.
38. Chatterjee S, Alampalli SV, Nageshan RK, Chettiar ST, Joshi S, Tatu US. Draft genome of a commonly misdiagnosed multidrug resistant pathogen Candida auris. BMC Genomics. 2015;16(1):686. doi: 10.1186/s12864-015-1863-z. PubMed PMID: 26346253; PMCID: PMC4562351.
39. Wang X, Bing J, Zheng Q, Zhang F, Liu J, Yue H, Tao L, Du H, Wang Y, Wang H, Huang G. The first isolate of Candida auris in China: clinical and biological aspects. Emerg Microbes Infect. 2018;7(1):93. doi: 10.1038/s41426-018-0095-0. PubMed PMID: 29777096; PMCID: PMC5959928.
40. Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13(1):122-43, table of contents. doi: 10.1128/CMR.13.1.122. PubMed PMID: 10627494; PMCID: PMC88936.
41. Gacser A, Trofa D, Schafer W, Nosanchuk JD. Targeted gene deletion in Candida parapsilosis demonstrates the role of secreted lipase in virulence. J Clin Invest. 2007;117(10):3049-58. doi: 10.1172/JCI32294. PubMed PMID: 17853941; PMCID: PMC1974868.
42. Trofa D, Soghier L, Long C, Nosanchuk JD, Gacser A, Goldman DL. A rat model of neonatal candidiasis demonstrates the importance of lipases as virulence factors for Candida albicans and Candida parapsilosis. Mycopathologia. 2011;172(3):169-78. doi: 10.1007/s11046-011-9429-3. PubMed PMID: 21667319.
43. Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, Long L, Isham N, Kovanda L, Borroto-Esoda K, Wring S, Angulo D, Ghannoum M. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother. 2017;61(5):e02396-16. doi: 10.1128/AAC.02396-16. PubMed PMID: 28223375; PMCID: PMC5404565.
44. Kean R, Delaney C, Sherry L, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R, Williams C, Ramage G. Transcriptome Assembly and Profiling of Candida auris Reveals Novel Insights into Biofilm-Mediated Resistance. mSphere. 2018;3(4):e00334-18. doi: 10.1128/mSphere.00334-18. PubMed PMID: 29997121; PMCID: PMC6041501.
45. Johnson CJ, Davis JM, Huttenlocher A, Kernien JF, Nett JE. Emerging Fungal Pathogen Candida auris Evades Neutrophil Attack. mBio. 2018;9(4). doi: 10.1128/mBio.01403-18. PubMed PMID: 30131360; PMCID: PMC6106086.
46. Wurster S, Bandi A, Beyda ND, Albert ND, Raman NM, Raad, II, Kontoyiannis DP. Drosophila melanogaster as a model to study virulence and azole treatment of the emerging pathogen Candida auris. J Antimicrob Chemother. 2019;74(7):1904-10. doi: 10.1093/jac/dkz100. PubMed PMID: 31225606; PMCID: PMC7967830.
47. Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, Liu X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J. 2019;17:661-74. doi: 10.1016/j.csbj.2019.03.006. PubMed PMID: 31205619; PMCID: PMC6558092.
48. Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Msd, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus - United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep. 2016;65(44):1234-7. doi: 10.15585/mmwr.mm6544e1. PubMed PMID: 27832049.
49. Wang Y, Xu J. Population genomic analyses reveal evidence for limited recombination in the superbug Candida auris in nature. Comput Struct Biotechnol J. 2022;20:3030-40. doi: 10.1016/j.csbj.2022.06.030. PubMed PMID: 35782746; PMCID: PMC9218166.
50. Bravo Ruiz G, Lorenz A. What do we know about the biology of the emerging fungal pathogen of humans Candida auris? Microbiol Res. 2021;242:126621. doi: 10.1016/j.micres.2020.126621. PubMed PMID: 33096325.
51. Chybowska AD, Childers DS, Farrer RA. Nine Things Genomics Can Tell Us About Candida auris. Front Genet. 2020;11:351. doi: 10.3389/fgene.2020.00351. PubMed PMID: 32351544; PMCID: PMC7174702.
52. Kumar D, Banerjee T, Pratap CB, Tilak R. Itraconazole-resistant Candida auris with phospholipase, proteinase and hemolysin activity from a case of vulvovaginitis. J Infect Dev Ctries. 2015;9(4):435-7. doi: 10.3855/jidc.4582. PubMed PMID: 25881537.
53. Kean R, Ramage G. Combined Antifungal Resistance and Biofilm Tolerance: the Global Threat of Candida auris. mSphere. 2019;4(4):e00458-19. doi: 10.1128/mSphere.00458-19. PubMed PMID: 31366705; PMCID: PMC6669339.
54. Kumar J, Eilertson B, Cadnum JL, Whitlow CS, Jencson AL, Safdar N, Krein SL, Tanner WD, Mayer J, Samore MH, Donskey CJ. Environmental Contamination with Candida Species in Multiple Hospitals Including a Tertiary Care Hospital with a Candida auris Outbreak. Pathog Immun. 2019;4(2):260-70. doi: 10.20411/pai.v4i2.291. PubMed PMID: 31768483; PMCID: PMC6827507.
55. Ledwoch K, Maillard J-Y. Candida auris Dry Surface Biofilm (DSB) for Disinfectant Efficacy Testing. Materials. 2019;12(1):18. PubMed PMID: doi: 10.3390/ma12010018.
56. Cadnum JL, Shaikh AA, Piedrahita CT, Sankar T, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. Effectiveness of Disinfectants Against Candida auris and Other Candida Species. Infect Control Hosp Epidemiol. 2017;38(10):1240-3. doi: 10.1017/ice.2017.162. PubMed PMID: 28793937.
57. Bapat PS, Nobile CJ. Photodynamic Therapy Is Effective Against Candida auris Biofilms. Front Cell Infect Microbiol. 2021;11:713092. doi: 10.3389/fcimb.2021.713092. PubMed PMID: 34540717; PMCID: PMC8446617.
58. Cadnum JL, Shaikh AA, Piedrahita CT, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. Relative Resistance of the Emerging Fungal Pathogen Candida auris and Other Candida Species to Killing by Ultraviolet Light. Infect Control Hosp Epidemiol. 2018;39(1):94-6. doi: 10.1017/ice.2017.239. PubMed PMID: 29157326.
59. Ghannoum MA, Rex JH, Galgiani JN. Susceptibility testing of fungi: current status of correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996;34(3):489-95. doi: 10.1128/jcm.34.3.489-495.1996. PubMed PMID: 8904400; PMCID: PMC228832.
60. Ghannoum M, Isham N, Angulo D, Borroto-Esoda K, Barat S, Long L. Efficacy of Ibrexafungerp (SCY-078) against Candida auris in an In Vivo Guinea Pig Cutaneous Infection Model. Antimicrob Agents Chemother. 2020;64(10):e00854-20. doi: 10.1128/AAC.00854-20. PubMed PMID: 32718958; PMCID: PMC7508588.
61. Pfaller MA, Diekema DJ, Ostrosky-Zeichner L, Rex JH, Alexander BD, Andes D, Brown SD, Chaturvedi V, Ghannoum MA, Knapp CC, Sheehan DJ, Walsh TJ. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J Clin Microbiol. 2008;46(8):2620-9. doi: 10.1128/JCM.00566-08. PubMed PMID: 18579718; PMCID: PMC2519503.
62. Huang X, Hurabielle C, Drummond RA, Bouladoux N, Desai JV, Sim CK, Belkaid Y, Lionakis MS, Segre JA. Murine model of colonization with fungal pathogen Candida auris to explore skin tropism, host risk factors and therapeutic strategies. Cell Host Microbe. 2021;29(2):210-21 e6. doi: 10.1016/j.chom.2020.12.002. PubMed PMID: 33385336; PMCID: PMC7878403.
63. Fakhim H, Vaezi A, Dannaoui E, Chowdhary A, Nasiry D, Faeli L, Meis JF, Badali H. Comparative virulence of Candida auris with Candida haemulonii, Candida glabrata and Candida albicans in a murine model. Mycoses. 2018;61(6):377-82. doi: 10.1111/myc.12754. PubMed PMID: 29460345.
64. Lionakis MS, Kontoyiannis DP. The growing promise of Toll-deficient Drosophila melanogaster as a model for studying Aspergillus pathogenesis and treatment. Virulence. 2010;1(6):488-99. doi: 10.4161/viru.1.6.13311. PubMed PMID: 21178494; PMCID: PMC3073358.
65. Chaabane F, Graf A, Jequier L, Coste AT. Review on Antifungal Resistance Mechanisms in the Emerging Pathogen Candida auris. Front Microbiol. 2019;10:2788. doi: 10.3389/fmicb.2019.02788. PubMed PMID: 31849919; PMCID: PMC6896226.
66. Rybak JM, Doorley LA, Nishimoto AT, Barker KS, Palmer GE, Rogers PD. Abrogation of Triazole Resistance upon Deletion of CDR1 in a Clinical Isolate of Candida auris. Antimicrob Agents Chemother. 2019;63(4):e00057-19. doi: 10.1128/AAC.00057-19. PubMed PMID: 30718246; PMCID: PMC6437491.
67. Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42(2):241-53. doi: 10.1128/AAC.42.2.241. PubMed PMID: 9527767; PMCID: PMC105395.
68. Healey KR, Kordalewska M, Jimenez Ortigosa C, Singh A, Berrio I, Chowdhary A, Perlin DS. Limited ERG11 Mutations Identified in Isolates of Candida auris Directly Contribute to Reduced Azole Susceptibility. Antimicrob Agents Chemother. 2018;62(10):e01427-18. doi: 10.1128/AAC.01427-18. PubMed PMID: 30082281; PMCID: PMC6153782.
69. Martins IM, Cortes JC, Munoz J, Moreno MB, Ramos M, Clemente-Ramos JA, Duran A, Ribas JC. Differential activities of three families of specific beta(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J Biol Chem. 2011;286(5):3484-96. doi: 10.1074/jbc.M110.174300. PubMed PMID: 21115488; PMCID: PMC3030354.
70. Dominguez EG, Zarnowski R, Choy HL, Zhao M, Sanchez H, Nett JE, Andes DR. Conserved Role for Biofilm Matrix Polysaccharides in Candida auris Drug Resistance. mSphere. 2019;4(1):e00680-18. doi: 10.1128/mSphereDirect.00680-18. PubMed PMID: 30602527; PMCID: PMC6315084.
71. Maphanga TG, Naicker SD, Kwenda S, Munoz JF, van Schalkwyk E, Wadula J, Nana T, Ismail A, Coetzee J, Govind C, Mtshali PS, Mpembe RS, Govender NP, for G-S. In Vitro Antifungal Resistance of Candida auris Isolates from Bloodstream Infections, South Africa. Antimicrob Agents Chemother. 2021;65(9):e0051721. doi: 10.1128/AAC.00517-21. PubMed PMID: 34228535; PMCID: PMC8370198.
72. Aghaei Gharehbolagh S, Izadi A, Talebi M, Sadeghi F, Zarrinnia A, Zarei F, Darmiani K, Borman AM, Mahmoudi S. New weapons to fight a new enemy: A systematic review of drug combinations against the drug-resistant fungus Candida auris. Mycoses. 2021;64(11):1308-16. doi: 10.1111/myc.13277. PubMed PMID: 33774879.
73. Jallow S, Govender NP. Ibrexafungerp: A First-in-Class Oral Triterpenoid Glucan Synthase Inhibitor. J Fungi (Basel). 2021;7(3):163. doi: 10.3390/jof7030163. PubMed PMID: 33668824; PMCID: PMC7996284.
74. Ghannoum M, Long L, Larkin EL, Isham N, Sherif R, Borroto-Esoda K, Barat S, Angulo D. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob Agents Chemother. 2018;62(6):e00244-18. doi: 10.1128/AAC.00244-18. PubMed PMID: 29610204; PMCID: PMC5971594.
Submitted August 2, 2022 | Accepted September 26, 2022 | Published October 21, 2022
Copyright © 2022 The Author(s). This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.