Dongyan Xu1, Thriveen S.C. Mana2, Jennifer L. Cadnum2, Abhishek Deshpande3, Faezeh Afsari4, Naseer Sangwan4, Curtis J. Donskey1,5
1Case Western Reserve University School of Medicine, Cleveland, Ohio
2Research Service, Cleveland VA Medical Center, Cleveland, Ohio
3Center for Value-Based Care Research, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio
4Lerner Research Institute/Lerner College of Medicine of Case Western Reserve University, Cleveland, Ohio
5Geriatric Research, Education and Clinical Center, Cleveland VA Medical Center, Cleveland, Ohio
Curtis J. Donskey
Infectious Diseases Section 1110W
Louis Stokes Cleveland VA Medical Center
10701 East Boulevard, Cleveland, Ohio 44106
Phone: 216-791-3800 ext. 64788
Fax: 216-229-8509
E-mail: Curtis.Donskey@va.gov
Xu D, Mana SCT, Cadnum JL, Deshpande A, Afsari F, Sangwan N, Donskey CJ. Why Does Doxycycline Pose a Relatively Low Risk for Promotion of Clostridioides difficile Infection? Pathogens and Immunity. 2022;7(1): 81–94. doi: 10.20411/pai.v7i1.512
10.20411/pai.v7i1.512
Background: Clinical studies suggest that doxycycline poses a low risk for promotion of Clostridioides difficile infection, but the microbiologic explanation for this finding is unclear.
Methods: Mice treated with oral doxycycline, oral azithromycin, subcutaneous ceftriaxone, doxycycline plus ceftriaxone, or azithromycin plus ceftriaxone were challenged with 104 colony-forming units of 2 different C. difficile strains on day 2 of 5 of treatment. The concentration of C. difficile was measured in stool 2 and 5 days after challenge. The impact of the treatments on the microbiota was assessed by sequencing.
Results: Doxycycline and azithromycin treatment did not promote colonization by either C. difficile strain in comparison to saline controls. Doxycycline treatment significantly reduced ceftriaxone-induced overgrowth of a C. difficile strain with doxycycline minimum-inhibitory concentration (MIC) of 0.06 µg/mL (P<0.01) but not a strain with doxycycline MIC of 48 µg/mL (P>0.05); azithromycin treatment did not reduce ceftriaxone-induced overgrowth of either strain. 16S rRNA amplicon sequencing revealed significantly lower bacterial diversity in the stool of ceftriaxone-treated mice, in comparison to doxycycline-treated and azithromycin-treated mice.
Conclusions: These findings suggest that doxycycline may have a low propensity to promote C. difficile colonization because it causes relatively limited alteration of the indigenous microbiota that provide colonization resistance and because it provides inhibitory activity against some C. difficile strains.
Clostridioides difficile; doxycycline; azithromycin; microbiome
Clostridioides difficile is the most important cause of healthcare-associated infectious diarrhea in developed countries [1]. Antibiotics that disrupt the indigenous intestinal microbiota are the major cause of C. difficile infection (CDI) [2–4]. Although all classes of antibiotics have been associated with CDI, cephalosporins, fluoroquinolones, clindamycin, and penicillins are generally considered the agents that pose the greatest risk [2–4]. To develop effective antimicrobial stewardship interventions for CDI, there is a need to identify antibiotics that have a relatively low propensity to disrupt the intestinal microbiota and cause CDI.
Doxycycline is an oral antibiotic used for treatment of a variety of conditions including community-acquired pneumonia, bronchitis, and soft tissue infections [2]. Several studies have reported that doxycycline may pose a relatively low risk for CDI [2, 5–8], but the microbiological explanation for this finding is unclear. One potential explanation is that doxycycline causes relatively little disruption of the indigenous intestinal microbiota [9, 10]. A second possibility is that doxycycline has activity against many C. difficile strains and might inhibit colonization if exposure occurs during therapy when the drug is present in the intestinal tract [3, 4, 6, 11, 12]. In the current study, we used an established mouse model to test the hypothesis that doxycycline has a relatively low risk for promotion of C. difficile colonization due to both limited disruption of the intestinal microbiota and inhibitory activity against C. difficile.
VA17 is a clinical epidemic North American pulsed-field gel electrophoresis type 1 (NAP1) C. difficile strain. And 386 is a restriction endonuclease analysis B2 toxin A and B positive C. difficile isolate with tetracycline minimum-inhibitory concentration (MIC) of 48 by Etest. C. difficile spores were prepared as previously described [13].
Broth dilution MICs of the test antibiotics for the test organisms were determined using standard methods for susceptibility testing of aerobic and anaerobic bacteria [14]. The concentrations of doxycycline and azithromycin in stool were determined by an agar diffusion assay with Bacillus subtilis as the indicator strain [14].
Fresh stool specimens were processed as described elsewhere [11, 12]. To quantify C. difficile, diluted samples were plated onto pre-reduced cycloserine-cefoxitin-brucella agar containing 0.1% taurocholic acid and 5 mg/mL lysozyme (C. difficile brucella agar), respectively. The plates were incubated for 48 hours, and the number of colony-forming units (CFU) of C. difficile per gram of sample was calculated.
The Animal Care Committee of the Cleveland Veterans Affairs Medical Center approved the experimental protocol. Female CF-1 mice (5 per group) weighing ~30 g (Harlan Sprague-Dawley, Indianapolis, IN) were housed in individual cages. Dose finding experiments were run to determine the amount of doxycycline to be dosed to result in stool concentrations in mice similar to those measured in humans (ie, mean 3.1 mg in stool on day 1 of treatment with 100 mg per day to human volunteers) [9]. Mice (5 per group) received a single oral administration of doxycycline in a dose equivalent to the usual human dose on a mg per kg basis (0.1 mg) or 5X (0.5 mg), 12X (1.2 mg), and 20X (2 mg) the usual human dose on a mg per kg basis. Fecal pellets were collected at 4, 8, and 24 hours after dosing. Fecal levels of doxycycline were measured by bioassay as described previously.
For azithromycin, we used a dose equivalent to the dose selected for doxycycline (5X the usual human dose on a mg per kg basis). For ceftriaxone, a dose equivalent to the usual human dose on a mg per kg basis was used because this dose has been used in previous mouse model studies and results in alteration of the microbiota of mice that is similar to alteration in ceftriaxone-treated humans [15].
Mice (5 per group) received 5 days of daily treatment with oral PBS (0.1 mL), doxycycline (0.5 mg in 0.1 mL PBS), oral azithromycin (1.25 mg in 0.1 mL PBS), subcutaneous ceftriaxone (1 mg in 0.1 mL PBS), subcutaneous ceftriaxone (1 mg) plus oral doxycycline (0.5 mg), or subcutaneous ceftriaxone (1 mg) plus oral azithromycin (1.25 mg). On day 2 of antibiotic treatment (4 to 6 hours after the antibiotic dose), mice received 10,000 CFU of C. difficile 368 or VA17 spores by orogastric gavage. The concentration of C. difficile in stool was measured 2 and 5 days after gavage of the spores. The rationale for administering azithromycin and doxycycline in combination with ceftriaxone was to determine if these agents have sufficient inhibitory activity to prevent ceftriaxone-induced overgrowth of C. difficile. For the VA17 strain, additional groups of mice were challenged with the VA17 strain during treatment with subcutaneous ceftriaxone plus 20X the usual human dose of doxycycline or azithromycin (ie, oral doxycycline 2 mg or oral azithromycin 5 mg).
Mice received daily dosing for 2 days with PBS (0.1 mL), doxycycline (0.5 mg or 5X the usual human dose in 0.1 mL PBS) by orogastric gavage, azithromycin (1.25 mg or 5X the usual human dose in 0.1 mL PBS) by orogastric gavage, or ceftriaxone (1 mg or 1X the usual human dose in 0.1 mL PBS) subcutaneously. Stool samples (~100 mg total) were collected 4 to 6 hours after the second daily dose for sequencing analysis.
DNA Extraction and 16S sRNA amplicon sequencing were performed as described previously [16]. Briefly, DNA was isolated from stool samples using the QIAamp DNA Microbiome kit (Qiagen). The isolated microbial gDNA was checked for signs of degradation and quantified using the Bio-analyzer (Agilent) to ensure accurate sample input for the initial PCR step. A nested PCR method was used for amplification of the V4 region of the 16S rRNA gene and the addition of Illumina Nextera Unique Dual indexes. Afterwards, each library underwent standard quality control procedures checking for sample concentration and sample quality. Each library was pooled together ensuring equal sample distribution amongst sequencing reads. Amplicon sequencing was performed on an Illumina MiSeq with a 2x150 read length.
One-way analysis of variance (ANOVA) was performed to compare concentrations of organisms among the treatment groups. P-values were adjusted for multiple comparisons using the Scheffe correction. Computations were performed with the use of Stata (version 5.0, Stata, College Station, Texas).
For analysis of the sequencing data, individual fastq files without non-biological nucleotides were processed using the Divisive Amplicon Denoising Algorithm (DADA) pipeline [17]. The output of the dada2 pipeline (feature table of amplicon sequence variants [an ASV table]) was processed for alpha and beta diversity analysis using phyloseq [18] and microbiomeSeq (http://www.github.com/umerijaz/microbiomeSeq) packages in R. Alpha diversity estimates were measured within group categories using estimate richness function of the phyloseq package. Canonical correspondence analysis (CCA) was performed using Bray-Curtis dissimilarity matrix between groups and visualized by using ggplot2 package [19]. Differential abundance analysis was performed using ANOVA in R software (The R Foundation for Statistical Computing, Vienna, Austria). As appropriate, we adjusted for multiple comparisons using the BH FDR method while performing multiple testing on taxa abundance across groups [20]. Permutational multivariate analysis of variance (PERMANOVA) was performed on all coordinates obtained during CCA.
For C. difficile VA17, the MICs of doxycycline, azithromycin, and ceftriaxone were 0.06, 16, and >64 µg/mL, respectively. For C. difficile 368, the MICs of doxycycline, azithromycin, and ceftriaxone were 48, 500, and >64 µg/mL, respectively.
No doxycycline was detected in the stool when doxycycline was administered as a single dose equivalent to the usual daily human dose. At 5X the usual daily human dose, doxycycline was detected at mean concentrations of 15.9 and 11.4 µg per mg stool at 4 and 8 hours post-dosing, respectively; no doxycycline was detected in samples collected 24 and 48 hours post-dosing. At 12X the usual human dose, doxycycline was detected at mean concentrations of 10.7, 26.3, and 5.1 µg per mg stool at 4, 8, and 24 hours post-dosing, respectively. At 20X the usual human dose, doxycycline was detected at mean concentrations of 14.6, 29.9, and 14.9 µg per mg stool at 4, 8, and 24 hours post-dosing, respectively. Based on these results and prior stool concentrations in humans, the 5X dose was chosen for subsequent testing.
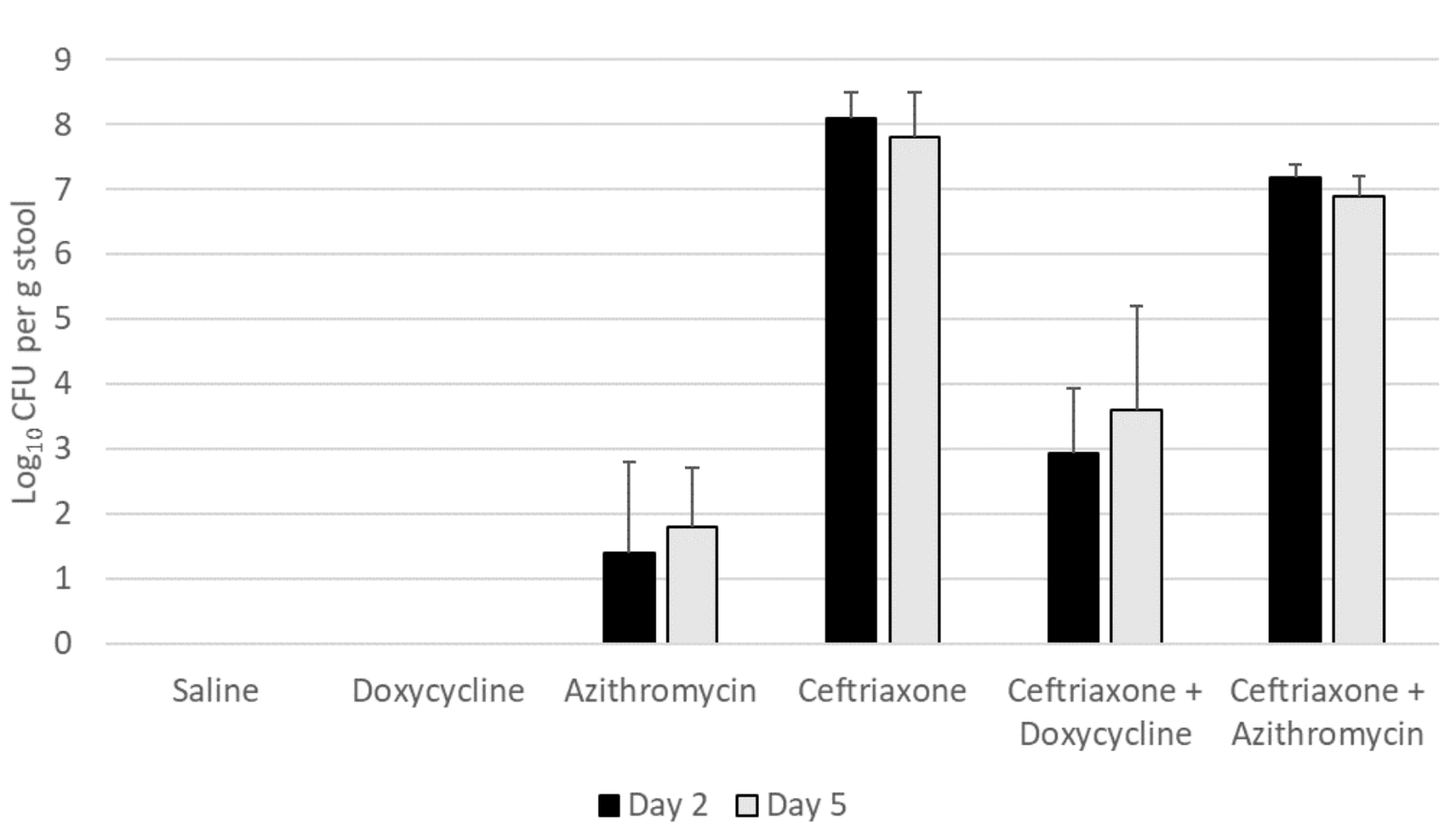
Figure 1A shows the impact of antibiotic treatment on establishment of C. difficile VA17 after exposure on day 2 of 5 of treatment. Ceftriaxone, ceftriaxone plus doxycycline, and ceftriaxone plus azithromycin promoted overgrowth of C. difficile VA17 in comparison to saline controls (P<0.05), whereas azithromycin (P=0.32) and doxycycline (P>.99) did not. In comparison to ceftriaxone-treated mice, ceftriaxone plus doxycycline-treated mice significantly reduced C. difficile concentrations (P<0.01), whereas ceftriaxone plus azithromycin-treated mice did not (P=0.09). When the dose of azithromycin and doxycycline was increased to 20-times the usual human dose on a mg per kg basis, ceftriaxone plus doxycycline-treated mice had no detectable C. difficile VA17 colonization, but all ceftriaxone plus azithromycin-treated mice had high-level colonization (>6.3 log10 CFU per g stool) (data not shown).
Figure 1B shows the impact of antibiotic treatment on establishment of C. difficile 368 after exposure on day 2 of 5 of treatment. Ceftriaxone, ceftriaxone plus doxycycline, and ceftriaxone plus azithromycin promoted overgrowth of C. difficile 368 in comparison to saline controls (P<0.05), whereas azithromycin and doxycycline did not (P>0.05). In comparison to ceftriaxone-treated mice, ceftriaxone plus doxycycline-treated mice and ceftriaxone plus azithromycin-treated mice had similar C. difficile concentrations (P>0.05).
Figure 1A. Doxycycline-susceptible Clostridioides difficile (strain VA17)

Figure 1B. Doxycycline-resistant Clostridioides difficile (strain 368)
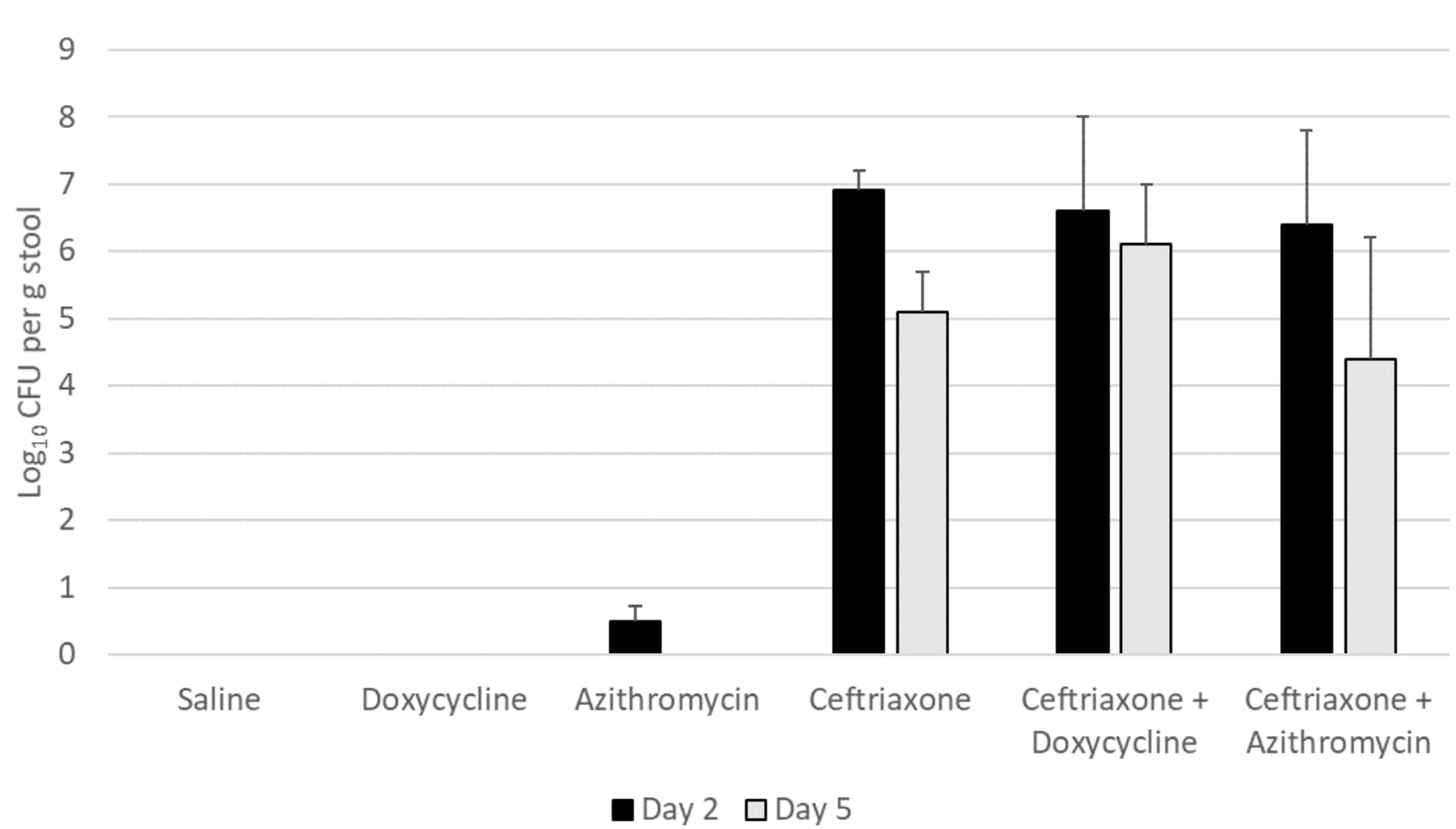
Figure 1: Effect of antibiotic treatment on establishment of colonization by Clostridioides difficile. Mice (5 per group) received 10,000 colony-forming units (CFU) of C. difficile VA17 (A) or 368 (B) on day 2 of 5 of daily antibiotic treatment. The concentration of C. difficile in stool was measured 2 days after gavage of the C. difficile test strain. Error bars represent standard error.
Figure 2 shows the impact of treatment on the total bacterial diversity in the stool of mice treated with normal saline, doxycycline, azithromycin, or ceftriaxone. Alpha (Simpson diversity index) and beta diversity (canonical correspondence analysis [CCA]) analysis of 16s rRNA gene amplicon sequencing data revealed differential community shift patterns in azithromycin, ceftriaxone, doxycycline, and PBS control samples. Doxycycline and azithromycin samples showed alpha diversity patterns similar to the PBS controls, suggesting that both antibiotics caused relatively limited disruption of the microbiota. In contrast, ceftriaxone treatment caused a marked (Wilcoxon Rank sum test, P<0.05) decrease in the alpha diversity as compared to doxycycline and azithromycin treatment. For ceftriaxone-treated mice, the relative percentage of Mucispirillum was substantially greater than in the other treatment groups, whereas the relative percentage of Alistipes was substantially reduced (Figure 2C).
Differential abundance analysis (P<0.05, ANOVA, Benjamini-Hochberg (BH]) highlighted 19 taxa at genus level whose abundance was differentially altered in the antibiotic treatment groups versus the PBS control group (Figure 3). In comparison to the PBS controls, ceftriaxone-treated mice had significantly increased abundance of Mucispirillum, Family XIII UCG-001, and Parvibacter, whereas the abundance of Alistipes, ASF 358, Family XIII AD3011, NK 4A214 group, Oscillibacter, and Tuzzerella was significantly decreased. In comparison to the PBS controls, doxycycline-treated mice had significantly reduced abundance of Family XIII UCG-001, Family XIII AD3011, Lachnospiraceae UCG-002, and Tuzzerella. In comparison to the PBS controls, azithromycin-treated mice had significantly increased abundance of Anaerovorax, Butyricicoccus, and Lachnospiraceae NK4A136 group, whereas the abundance of Family XIII AD3011 was significantly decreased.
Figure 2A
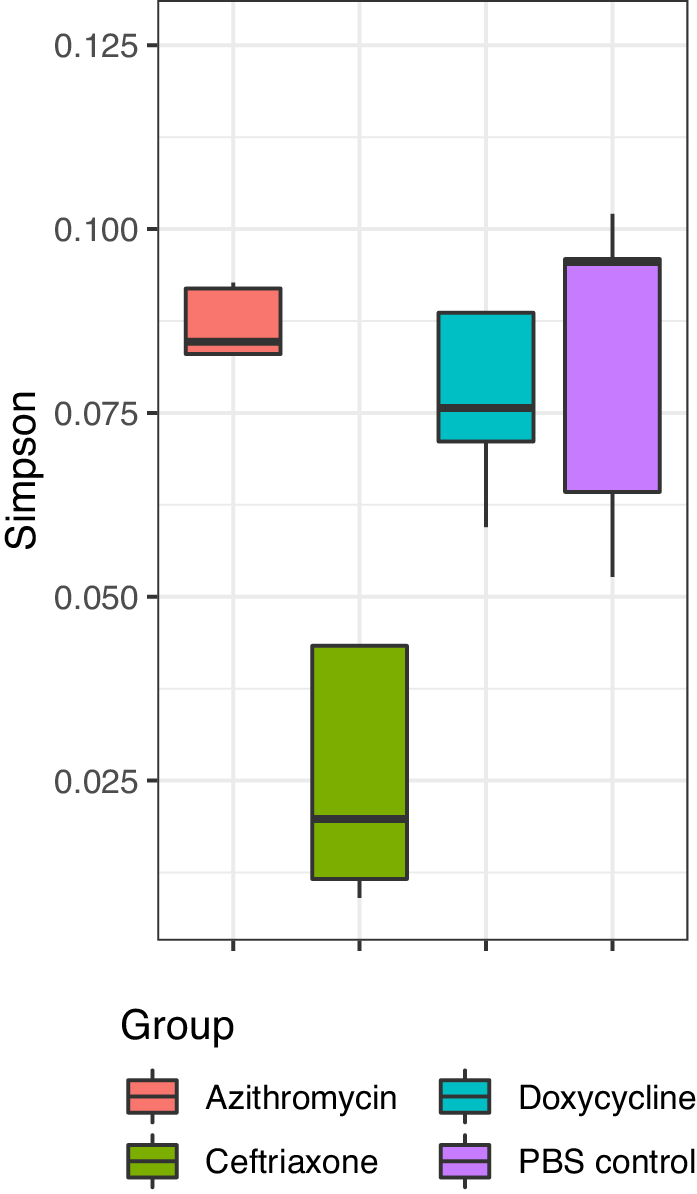
Figure 2B
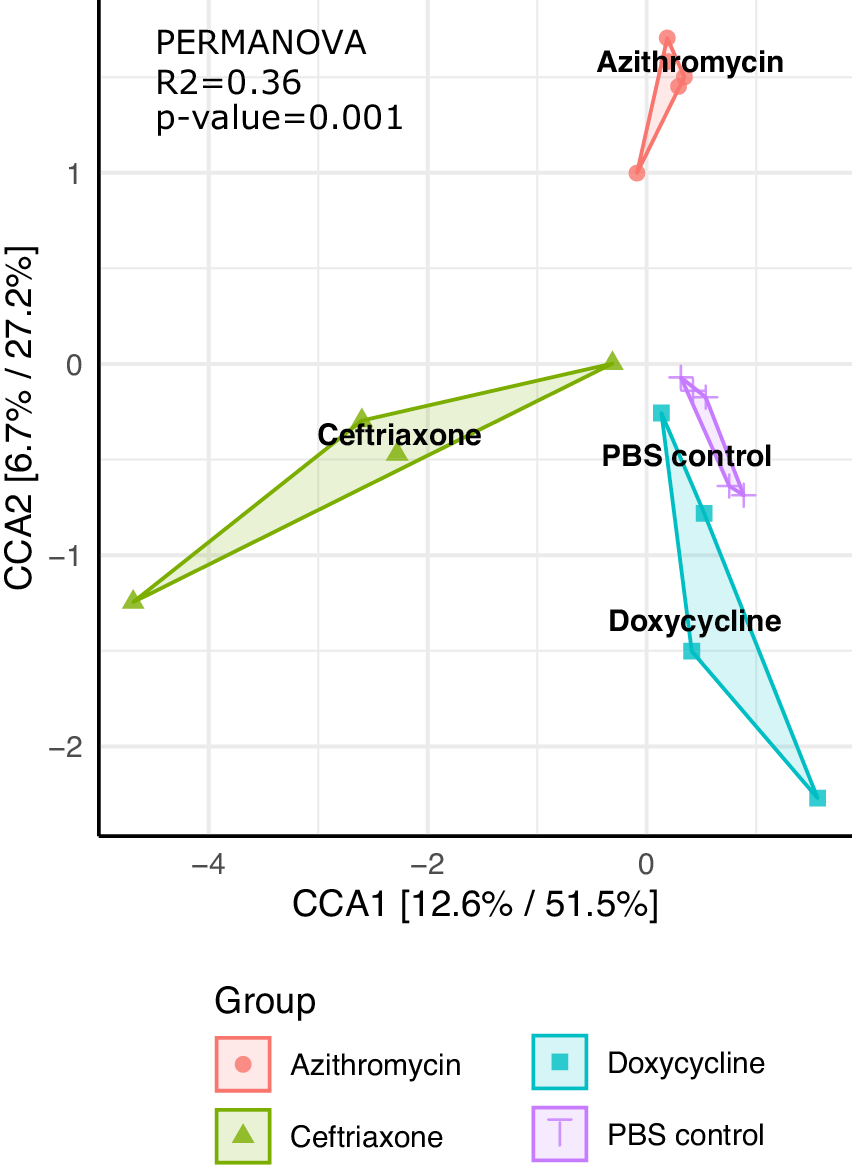
Figure 2C
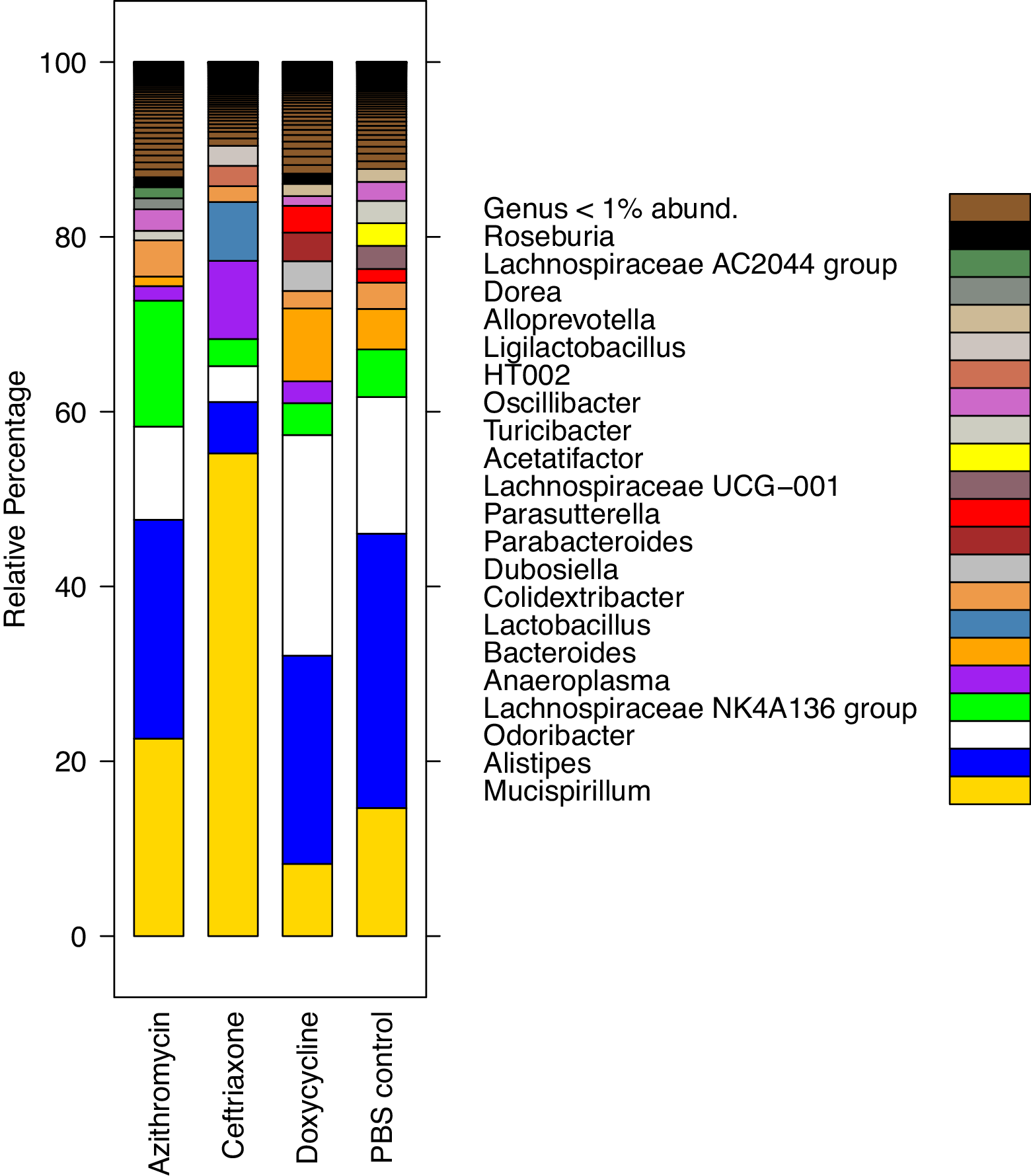
Figure 2. Impact of treatment with phosphate-buffered saline (PBS), doxycycline, azithromycin, or ceftriaxone on the total bacterial diversity in the stool of mice. A) Alpha Simpson diversity index; B) Beta diversity canonical correspondence analysis (CCA); C) Relative percentage abundance of different bacterial taxa. Black horizontal lines associated with the box plots represent median values.
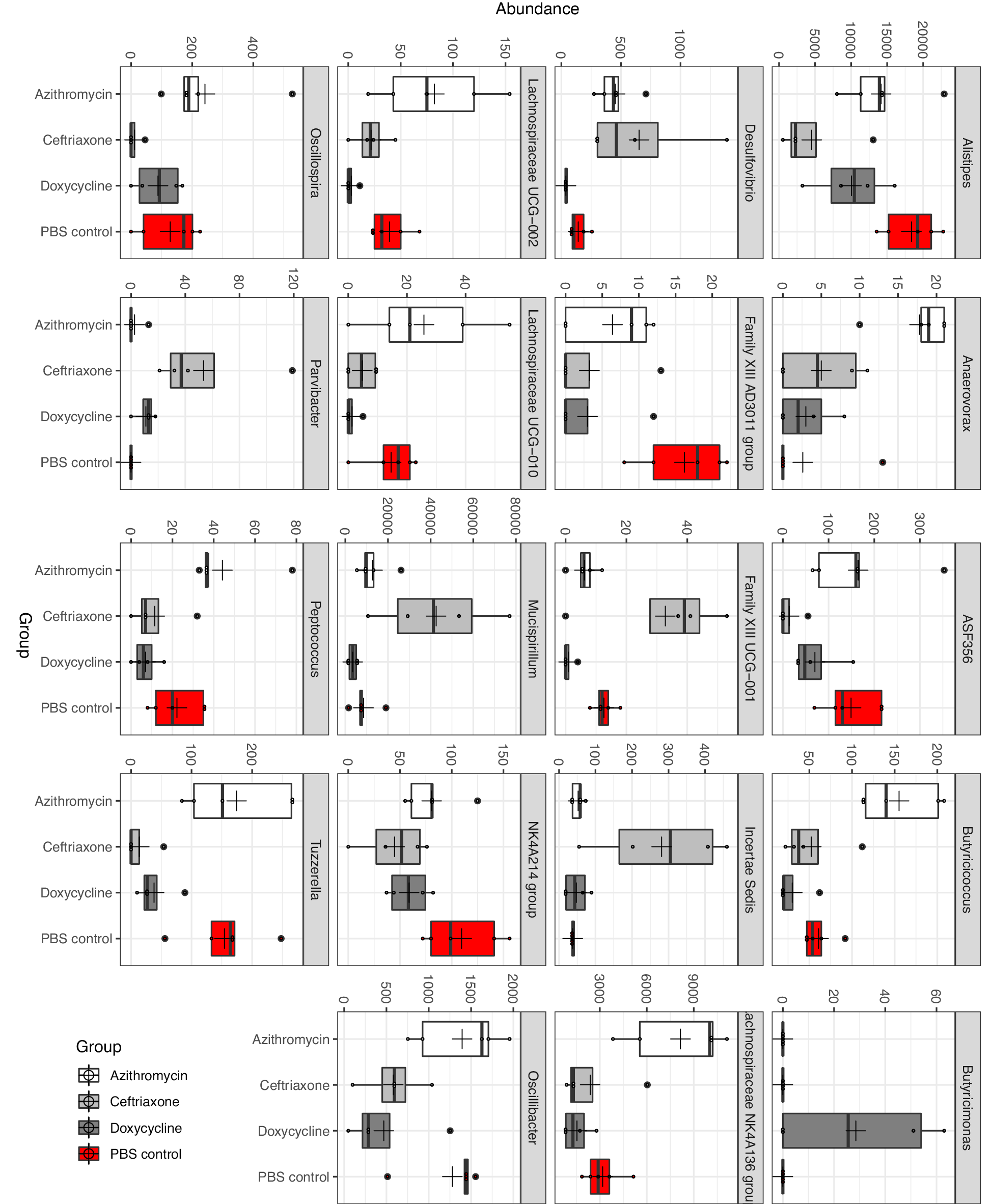
Figure 3. Differential abundance analysis (P<0.05, ANOVA, Benjamini-Hochberg (BH)) highlighting 19 taxa at genus level whose abundance was differentially altered in the antibiotic treatment groups versus the phosphate-buffered saline (PBS) control group.
In a mouse model, treatment with oral doxycycline did not promote colonization by doxycycline-susceptible or doxycycline-resistant strains of C. difficile. Doxycycline administered in combination with ceftriaxone reduced ceftriaxone-induced overgrowth of the susceptible but not the resistant strain of C. difficile, demonstrating that doxycycline achieved sufficient concentrations in the intestinal tract to reduce colonization by the doxycycline-susceptible C. difficile strain. These results suggest that doxycycline may have a low propensity to promote C. difficile both because it causes relatively little alteration of the indigenous microbiota that provide colonization resistance against C. difficile, and because it provides inhibitory activity against some strains of C. difficile.
Our findings are consistent with previous evidence that doxycycline treatment can alter the intestinal microbiota, but to a relatively modest degree in comparison to antibiotics such as ceftriaxone [9, 10, 21–23]. Although emergence of doxycycline-resistant microorganisms was a common finding in studies of healthy volunteers, only relatively minor alterations in the concentration of different components of the microbiota were reported based on culture results [9, 10, 21]. Doxycycline treatment also only minimally altered short-chain fatty acid and bile pigment excretion [21, 22].
In recent surveillance studies, a majority of C. difficile isolates have been susceptible to tetracyclines [4, 6, 7]. In a recent systematic review and meta-analysis of antimicrobial resistance in C. difficile, tetracycline had a calculated weighted pooled resistance of 20% [7]. These findings suggest that doxycycline may have sufficient activity to inhibit many currently circulating strains of C. difficile. Macrolide antibiotics also have variable activity against C. difficile [4], and therefore we cannot exclude the possibility that azithromycin might inhibit some circulating C. difficile strains.
Given the evidence that doxycycline may have a low propensity to promote CDI, it has been proposed that doxycycline might be preferred over azithromycin for treatment of community-acquired pneumonia [6, 7, 24]. In clinical studies, macrolides have been associated with an increased risk for CDI in comparison to tetracyclines [2, 5–8]. In the current study, neither drug promoted significant overgrowth of C. difficile, although low levels of C. difficile were detected in the stool of azithromycin-treated but not doxycycline-treated mice. Additional studies are needed to determine if doxycycline has a relatively low propensity to promote intestinal colonization with other healthcare-associated pathogens. In mice, doxycycline did not promote colonization by vancomycin-resistant enterococci (VRE) or Klebsiella pneumoniae, while azithromycin promoted VRE but not K. pneumoniae (author’s unpublished data).
One notable finding from the sequencing analysis was that ceftriaxone treatment resulted in a significant increase in the proportion of Mucispirillum spp. Mucispirillum schaedleri (phylum Deferribacteres) is present in the intestinal microbiota of mice and other animals and is a low-abundance member of the human intestinal microbiota associated with the intestinal mucosa [25, 26]. M. schaedleri has been shown to protect mice against enteric Salmonella enterica serovar Typhimurium infection by interfering with pathogen invasion and virulence factor expression [25]. Additional studies are needed to determine if M. schaedleri plays a role in prevention of colonization by healthcare-associated pathogens such as C. difficile.
Our study has some limitations. We studied only 2 strains of C. difficile. The study was conducted using a mouse model with healthy mice dosed once daily with the antibiotics. Although levels of doxycycline in stool samples were similar to levels previously reported in humans [9, 10, 23], antibiotic excretion in the intestinal tract of mice and humans may differ. Therefore, additional studies will be required to confirm that the findings are applicable to patients. Finally, the challenge with pathogens occurred during antibiotic treatment. Antibiotic-induced disruption of the microbiota may result in a vulnerable period for establishment of colonization after completion of antibiotic treatment [27]. Further studies are needed to assess establishment of colonization when pathogen challenge occurs after completion of treatment.
This work was supported by the Department of Veterans Affairs. We thank Dr. Dale Gerding for providing C. difficile isolate 386. The authors declare that no competing interests exist.
This work was supported by the Department of Veterans’ Affairs (Merit Review grant no. CX001848 to CJD).
CJD has received research funding from Clorox, Pfizer, and PDI. All other authors report no conflicts of interest relevant to this article.
1. Guh AY, Mu Y, Winston LG, Johnston H, Olson D, Farley MM, Wilson LE, Holzbauer SM, Phipps EC, Dumyati GK, Beldavs ZG, Kainer MA, Karlsson M, Gerding DN, McDonald LC, Emerging Infections Program Clostridioides difficile Infection Working G. Trends in U.S. Burden of Clostridioides difficile Infection and Outcomes. N Engl J Med. 2020;382(14):1320-30. doi: 10.1056/NEJMoa1910215. PubMed PMID: 32242357; PMCID: PMC7861882.
2. Deshpande A, Pasupuleti V, Thota P, Pant C, Rolston DD, Sferra TJ, Hernandez AV, Donskey CJ. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68(9):1951-61. doi: 10.1093/jac/dkt129. PubMed PMID: 23620467.
3. Donskey CJ, Kundrapu S, Deshpande A. Colonization versus carriage of Clostridium difficile. Infect Dis Clin North Am. 2015;29(1):13-28. doi: 10.1016/j.idc.2014.11.001. PubMed PMID: 25595843.
4. Owens RC, Jr., Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46 Suppl 1:S19-31. doi: 10.1086/521859. PubMed PMID: 18177218.
5. Brown KA, Langford B, Schwartz KL, Diong C, Garber G, Daneman N. Antibiotic Prescribing Choices and Their Comparative C. Difficile Infection Risks: A Longitudinal Case-Cohort Study. Clin Infect Dis. 2021;72(5):836-44. doi: 10.1093/cid/ciaa124. PubMed PMID: 32069358; PMCID: PMC7935390.
6. Doernberg SB, Winston LG, Deck DH, Chambers HF. Does doxycycline protect against development of Clostridium difficile infection? Clin Infect Dis. 2012;55(5):615-20. doi: 10.1093/cid/cis457. PubMed PMID: 22563022; PMCID: PMC3491851.
7. Tariq R, Cho J, Kapoor S, Orenstein R, Singh S, Pardi DS, Khanna S. Low Risk of Primary Clostridium difficile Infection With Tetracyclines: A Systematic Review and Metaanalysis. Clin Infect Dis. 2018;66(4):514-22. doi: 10.1093/cid/cix833. PubMed PMID: 29401273.
8. Tartof SY, Rieg GK, Wei R, Tseng HF, Jacobsen SJ, Yu KC. A Comprehensive Assessment Across the Healthcare Continuum: Risk of Hospital-Associated Clostridium difficile Infection Due to Outpatient and Inpatient Antibiotic Exposure. Infect Control Hosp Epidemiol. 2015;36(12):1409-16. doi: 10.1017/ice.2015.220. PubMed PMID: 26387888.
9. Nord CE, Heimdahl A. Impact of orally administered antimicrobial agents on human oropharyngeal and colonic microflora. J Antimicrob Chemother. 1986;18 Suppl C:159-64. doi: 10.1093/jac/18.supplement_c.159. PubMed PMID: 3804892.
10. Rashid MU, Panagiotidis G, Backstrom T, Weintraub A, Nord CE. Ecological impact of doxycycline at low dose on normal oropharyngeal and intestinal microflora. Int J Antimicrob Agents. 2013;41(4):352-7. doi: 10.1016/j.ijantimicag.2012.11.014. PubMed PMID: 23332619.
11. Adams DA, Riggs MM, Donskey CJ. Effect of fluoroquinolone treatment on growth of and toxin production by epidemic and nonepidemic clostridium difficile strains in the cecal contents of mice. Antimicrob Agents Chemother. 2007;51(8):2674-8. doi: 10.1128/AAC.01582-06. PubMed PMID: 17562807; PMCID: PMC1932513.
12. Kundrapu S, Sunkesula VC, Jury LA, Cadnum JL, Nerandzic MM, Musuuza JS, Sethi AK, Donskey CJ. Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis. 2016;16:159. doi: 10.1186/s12879-016-1514-2. PubMed PMID: 27091232; PMCID: PMC4835867.
13. Tomas ME, Mana TSC, Wilson BM, Nerandzic MM, Joussef-Pina S, Quinones-Mateu ME, Donskey CJ. Tapering Courses of Oral Vancomycin Induce Persistent Disruption of the Microbiota That Provide Colonization Resistance to Clostridium difficile and Vancomycin-Resistant Enterococci in Mice. Antimicrob Agents Chemother. 2018;62(5). doi: 10.1128/AAC.02237-17. PubMed PMID: 29530853; PMCID: PMC5923165.
14. Wayne PA. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: CLSI; 2011 [cited 32(3) M100-S20].
15. Hoyen CK, Pultz NJ, Paterson DL, Aron DC, Donskey CJ. Effect of parenteral antibiotic administration on establishment of intestinal colonization in mice by Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 2003;47(11):3610-2. doi: 10.1128/AAC.47.11.3610-3612.2003. PubMed PMID: 14576127; PMCID: PMC253805.
16. Helsley RN, Miyata T, Kadam A, Varadharajan V, Sangwan N, Huang EC, Banerjee R, Brown AL, Fung KK, Massey WJ, Neumann C, Orabi D, Osborn LJ, Schugar RC, McMullen MR, Bellar A, Poulsen KL, Kim A, Pathak V, Mrdjen M, Anderson JT, Willard B, McClain CJ, Mitchell M, McCullough AJ, Radaeva S, Barton B, Szabo G, Dasarathy S, Garcia-Garcia JC, Rotroff DM, Allende DS, Wang Z, Hazen SL, Nagy LE, Brown JM. Gut microbial trimethylamine is elevated in alcohol-associated hepatitis and contributes to ethanol-induced liver injury in mice. Elife. 2022;11. doi: 10.7554/eLife.76554. PubMed PMID: 35084335; PMCID: PMC8853661.
17. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581-3. doi: 10.1038/nmeth.3869. PubMed PMID: 27214047; PMCID: PMC4927377.
18. McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. doi: 10.1371/journal.pone.0061217. PubMed PMID: 23630581; PMCID: PMC3632530.
19. Wickham H. ggplot2: Elegant Graphics for Data Analysis. springer; 2016.
20. Benjamini Y. Discovering the false discovery rate. Journal of the Royal Statistical Society: Series B (Statistical Methodology). 2010;72(4):405-16.
21. Hoverstad T, Carlstedt-Duke B, Lingaas E, Norin E, Saxerholt H, Steinbakk M, Midtvedt T. Influence of oral intake of seven different antibiotics on faecal short-chain fatty acid excretion in healthy subjects. Scand J Gastroenterol. 1986;21(8):997-1003. doi: 10.3109/00365528608996411. PubMed PMID: 3775265.
22. Saxerholt H, Carlstedt-Duke B, Hoverstad T, Lingaas E, Norin KE, Steinbakk M, Midtvedt T. Influence of antibiotics on the faecal excretion of bile pigments in healthy subjects. Scand J Gastroenterol. 1986;21(8):991-6. doi: 10.3109/00365528608996410. PubMed PMID: 3775264.
23. Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1(2):101-14. doi: 10.1016/S1473-3099(01)00066-4. PubMed PMID: 11871461.
24. Di Bella S, Taglietti F, Petrosillo N. Are there reasons to prefer tetracyclines to macrolides in older patients with community-acquired pneumonia? Antimicrob Agents Chemother. 2013;57(8):4093. doi: 10.1128/AAC.00828-13. PubMed PMID: 23858063; PMCID: PMC3719784.
25. Herp S, Brugiroux S, Garzetti D, Ring D, Jochum LM, Beutler M, Eberl C, Hussain S, Walter S, Gerlach RG, Ruscheweyh HJ, Huson D, Sellin ME, Slack E, Hanson B, Loy A, Baines JF, Rausch P, Basic M, Bleich A, Berry D, Stecher B. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe. 2019;25(5):681-94 e8. doi: 10.1016/j.chom.2019.03.004. PubMed PMID: 31006637.
26. Herp S, Durai Raj AC, Salvado Silva M, Woelfel S, Stecher B. The human symbiont Mucispirillum schaedleri: causality in health and disease. Med Microbiol Immunol. 2021;210(4):173-9. doi: 10.1007/s00430-021-00702-9. PubMed PMID: 34021796.
27. Abujamel T, Cadnum JL, Jury LA, Sunkesula VC, Kundrapu S, Jump RL, Stintzi AC, Donskey CJ. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One. 2013;8(10):e76269. doi: 10.1371/journal.pone.0076269. PubMed PMID: 24098459; PMCID: PMC3788714.
Submitted March 27, 2022 | Accepted May 19, 2022 | Published June 21, 2022
Copyright © 2022 Pathogens and Immunity. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.