Aline I. Moser1, Peter M. Keller,1 Edgar I. Campos-Madueno,1,2 Laurent Poirel,3,4,5 Patrice Nordmann,3,4,5 and Andrea Endimiani1*
1 Institute for Infectious Diseases (IFIK), University of Bern, Bern, Switzerland
2 Graduate School of Cellular and Biomedical Sciences, University of Bern, Bern, Switzerland
3 Emerging Antibiotic Resistance Unit, Medical and Molecular Microbiology, Department of Medicine, University of Fribourg, Fribourg, Switzerland
4 French INSERM European Unit, University of Fribourg (LEA-IAME), Fribourg, Switzerland
5 National Reference Center for Emerging Antibiotic Resistance (NARA), Fribourg, Switzerland
Prof. Andrea Endimiani MD, PhD
Institute for Infectious Diseases, University of Bern
Friedbühlstrasse 51, CH-3001, Bern, Switzerland
Phone: +41-31-632 8 632; Fax: +41-31-632 8 766
Emails: andrea.endimiani@ifik.unibe.ch; aendimiani@gmail.com
Moser AI, Keller PM, Campos-Madueno EI, Poirel L, Nordmann P, Endimiani A. A patient with multiple carbapenemase producers including an unusual Citrobacter sedlakii hosting an IncC blaNDM-1- and armA-carrying plasmid. Pathogens and Immunity. 2021;6(2): 119–134. doi: 10.20411/pai.v6i2.482
10.20411/pai.v6i2.482
Background. Patients colonized with multiple species of carbapenemase-producing Enterobacterales (CPE) are increasingly observed. This phenomenon can be due to the high local prevalence of these pathogens, the presence of important host risk factors, and the great genetic promiscuity of some carbapenemase genes.
Methods. We analyzed 4 CPE (Escherichia coli, Klebsiella pneumoniae, Providencia stuartii, Citrobacter sedlakii), 1 extended-spectrum cephalosporin-resistant K. pneumoniae (ESC-R-Kp), and 1 carbapenemase-producing Acinetobacter baumannii simultaneously isolated from a patient transferred from Macedonia. Susceptibility tests were performed using a microdilution MIC system. The complete genome sequences were obtained by using both short-read and long-read whole-genome sequencing technologies.
Results. All CPE presented high-level resistance to all aminoglycosides due to the expression of the armA 16S rRNA methylase. In C. sedlakii and E. coli (ST69), both the carbapenemase blaNDM-1 and armA genes were located on an identical IncC plasmid of type 1a. The K. pneumoniae (ST268) and P. stuartii carried chromosomal blaNDM-1 and blaOXA-48, respectively, while the ESC-R-Kp (ST395) harbored a plasmid-located blaCTX-M-15. In the latter 3 isolates, armA-harboring IncC plasmids similar to plasmids found in C. sedlakii and E. coli were also detected. The A. baumannii strain possessed the blaOXA-40 carbapenemase gene.
Conclusions. The characterization of the genetic organization of IncC-type plasmids harbored by 3 different species from the same patient offered insights into the evolution of these broad- host-range plasmids. Moreover, we characterized here the first complete genome sequence of a carbapenemase-producing C. sedlakii strain, providing a reference for future studies on this rarely reported species.
carbapenemases, NDM-1, Enterobacterales, ArmA, plasmid, CPE
The spread of carbapenemase-producing Enterobacterales (CPE) represents a major public health issue. To date, KPC-2/-3- and OXA-48-producing Escherichia coli and Klebsiella pneumoniae isolates have been reported worldwide and in some geographic areas their prevalence is alarming [1, 2]. In addition, though less predominant, the NDM-producing species are of particular clinical concern because the NDM carbapenemase activity cannot be inhibited by clinically available β-lactamase inhibitors [3]. Moreover, the blaNDM genes show great promiscuity since they can be located in different genetic environments, being either integrated into the chromosome or on extra-chromosomal mobile genetic elements (MGEs) among different bacterial species. In particular, some conjugative plasmids harbor additional antimicrobial resistance genes (ARGs) conferring co-resistances to other antibiotic families, such as the ArmA 16S rRNA methylase enzyme that modifies the target of aminoglycosides resulting in resistance to all clinically-used aminoglycosides, including the most recently developed plazomicin [4–6].
In this overall scenario, reports of patients simultaneously infected and/or colonized with multiple species of CPE are becoming a source of real concern. Several cases of interspecies exchange of identical blaKPC- [7, 8], blaOXA-48- [9, 10], and blaNDM-1-carrying plasmids have been described [9, 11–13]. In particular, those involving the blaNDM-1 were mainly due to the horizontal spread of broad-host-range IncC plasmids (formerly IncA/C2) [14]. In such cases, 2 to 4 different CPE were isolated from the same subjects: E. coli and K. pneumoniae were usually involved in this phenomenon, but Klebsiella oxytoca, Citrobacter freundii, Proteus mirabilis, or Morganella morganii strains could also be encountered [9, 11, 13].
In this work, we report a clinical case of a patient being simultaneously colonized by 3 blaNDM-1- and one blaOXA-48-positive Enterobacterales, along with an Acinetobacter baumannii strain possessing a blaOXA-40 carbapenemase gene. Enterobacterales were characterized at the genomic level by implementing both short-read and long-read whole-genome sequencing (WGS) technologies. Above all, we provide here the first genomic characterization of a unique blaNDM-1- and armA-positive Citrobacter sedlakii isolate.
Clinical case. In December 2020, a Swiss man in his 20s was admitted at the Inselspital (Bern, Switzerland). The subject was transferred from Macedonia, where he had been hospitalized as a polytraumatized individual for 2 months (further detailed clinical data regarding this hospitalization are not available). For surveillance purpose, a rectal swab to screen for the presence of multidrug-resistant Gram-negative bacteria was withdrawn. Moreover, blood cultures, swabs from skin ulcers, and cultures from vascular catheters were also performed over the course of 14 days (Table 1). The patient was kept in isolation during these 2 weeks before transfer to another Swiss hospital. The present anonymized case description has been carried out in accordance with the Declaration of Helsinki. The patient has also signed a general consent.
Species identification (ID) and antimicrobial susceptibility tests (ASTs). ID was routinely obtained using the matrix assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS; Bruker); it was then achieved using WGS data and the implementation of the Type (Strain) Genome Server (https://tygs.dsmz.de/). ASTs were performed using the broth microdilution ESB1F and GNX2F Sensititre panels (Thermo Scientific). Minimum inhibitory concentrations (MICs) for antibiotics were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria (version 9.0, 2019).
Detection of ESBL and carbapenemase-producing (CP) strains. The Rapid ESBL NP, Carba NP, and CarbAcineto NP colorimetric tests, along with the NG-Test CARBA-5 and the eazyplex Superbug complete B assays, were used to screen strains showing reduced susceptibility to extended-spectrum cephalosporins (ESCs) and/or carbapenems [1, 15]. Enterobacterales were further characterized by implementing the WGS (see below), whereas the A. baumannii was typed with a PCR/sequencing approach [16].
Whole-genome sequencing (WGS). Both NovaSeq 6000 (NEBNext Ultra II DNA library prep kit for Illumina; 2 x 150-bp paired-end reads) and MinION (SQK-RBK004 library; FLO-MIN 106D R9 flow-cell; Oxford Nanopore) technologies were implemented to perform WGS as previously described with an average sequencing coverage of 190x [17, 18]. In short, sequencing adapters from both Illumina and Nanopore reads were removed using Trimmomatic (v0.36) and Porechop (v0.2.4), respectively. The hybrid assembly was generated using Unicycler (v0.4.8) with default settings. Annotation was performed with the NCBI pipeline, but insertion sequences (ISs) were manually curated with ISfinder (https://isfinder.biotoul.fr/). The final genome was analyzed using the overall tools of the Center for Genomic Epidemiology (www.genomicepidemiology.org/). Integrons were classified according to INTEGRALL (http://integrall.bio.ua.pt/). The average nucleotide identity (ANI) was calculated using the OrthoANIu Calculator (http://www.ezbiocloud.net/tools/ani).
The complete genome assemblies of the 5 Enterobacterales have been deposited in GenBank (CP071068-CP071089) under BioProject PRJNA698767.
Samples and bacteria. Numerous samples taken at the admission of the patient gave positive results for CPE (E. coli, K. pneumoniae, C. freundii complex, and Providencia stuartii) and for a CP A. baumannii. An ESC-resistant K. pneumoniae (ESC-R-Kp) and a carbapenem-resistant P. aeruginosa were also isolated in multiple specimens (Table 1).
To study the features of these MDR-Gram-negatives, 4 representative strains of the CPE species, the ESC-R-Kp, and the CP A. baumannii were selected for further phenotypic and molecular analyses. The antibiotic MICs for these 6 illustrative strains are depicted in Table 2. As expected, the 5 CP strains showed reduced susceptibility to carbapenems, but the 4 CPE also presented high-level resistance to all tested aminoglycosides. Moreover, the ESC-R-Kp showed a phenotype consistent with the extended-spectrum ß-lactamase (ESBL) production.
Molecular features of the MDR bacteria. As shown in Table 3, the gut flora of the patient was colonized with a sequence type (ST) 69 E. coli strain (named 3347558) possessing numerous ARGs, including the carbapenemase gene blaNDM-1, the ESBL gene blaCTX-M-15, and the 16S rRNA methylase gene armA. Of note, the pandemic ST69 lineage is rarely associated with blaNDM-1, and it has never been reported to contain simultaneously both blaNDM-1 and armA [19].
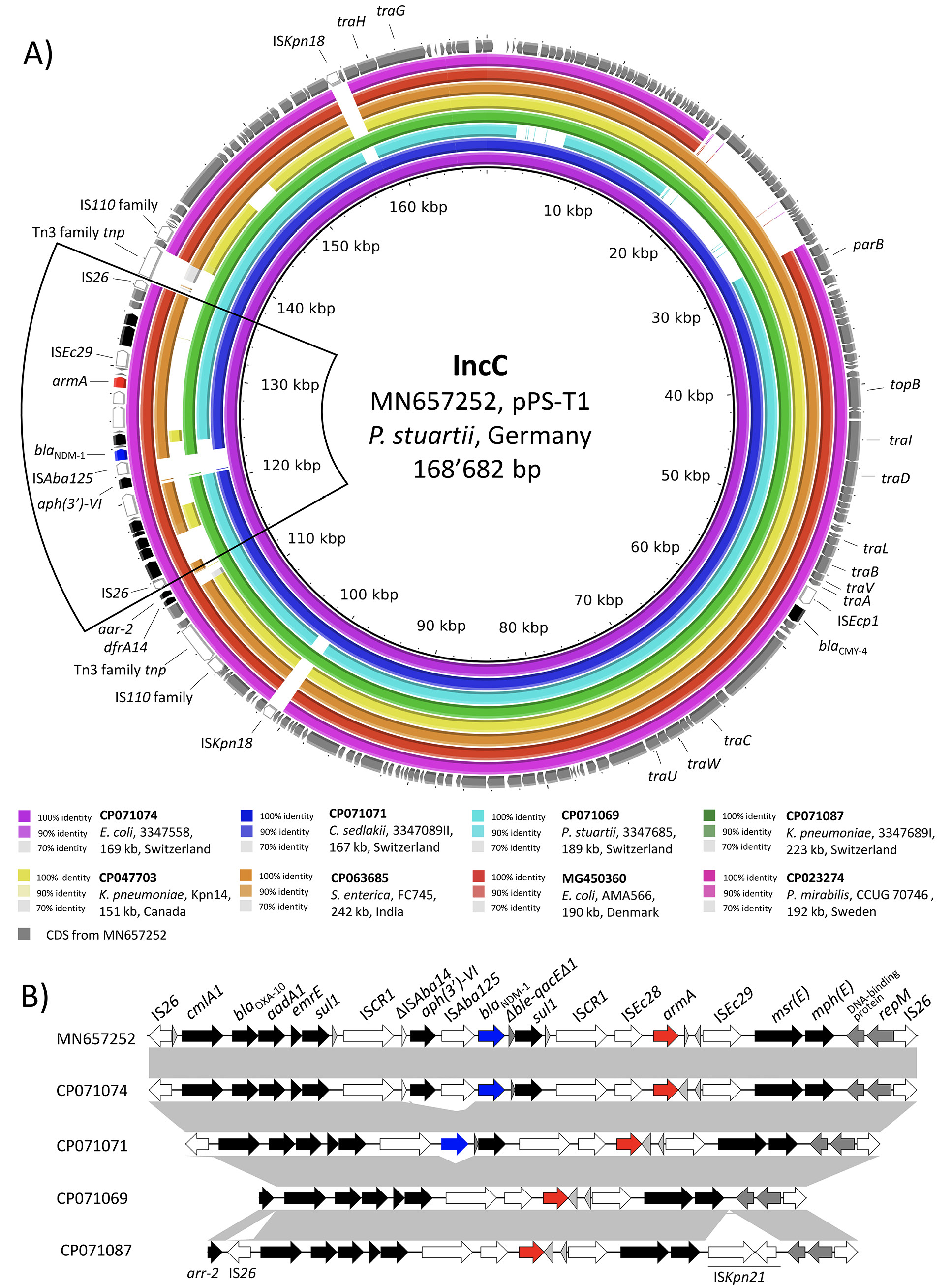
In strain 3347558, blaNDM-1 and armA were co-carried on the ARI-A island of a multidrug resistance 169kb IncC plasmid of type 1a (p33477558_1) identical (identity, 99.81%) to pPS-T1 found in Germany (2015) in a P. stuartii strain of human origin (Figure 1A) [20]. In both p33477558_1 and pPS-T1, the blaNDM-1 was associated with ISAba125 and located between 2 ISCR1 elements in a genetic environment identical to the one reported for the Serbian P. aeruginosa isolate MMA83 [4, 5, 21, 22]. Moreover, armA was positioned upstream of blaNDM-1, and it was organized in a classic element (ISEc28-armA-ISEc29) (Figure 1B) [23].
The patient also carried 2 K. pneumoniae strains belonging to different STs (Table 3).

Figure 1. BLASTn comparison of IncC-type plasmid sequences. A) The IncC-type plasmids from the present study were compared to other sequences selected based on high homology in a BLASTn search against the NCBI non-redundant nucleotide collection. Plasmid sequence pPS-T1 was used as reference sequence. Rings were constructed using BRIG (BLAST Ring Image Generator) v.0.95. The colored rings represent similarities to the reference sequence. CDS are depicted as grey arrows in the outermost circle. Mobile genetic elements (MGE) are depicted in white, the blaNDM-1 in blue, armA in red, and all other antimicrobial resistance genes (ARGs) in black. We report GenBank accession, species of isolation, strain name, sequence size, and country of origin. The blaNDM-1-containing region is bordered with a black line. B) Genetic environment of the blaNDM-1- and armA-containing region in the reference sequence pPS-T1 (GenBank: MN657252) compared to the IncC plasmids from our study. Hypothetical proteins are depicted as light grey arrows, other CDS are depicted in dark grey.
The ESC-R-Kp strain 3347684 II was of ST268 and possessed a non-typeable plasmid of 64kb that carried the blaCTX-M-15. In contrast, the CP-Kp 3347689 I was of ST395 and possessed a chromosomally located blaNDM-1 along with a multidrug resistance 223kb multi-replicon IncC/R plasmid (p3347689I_1) carrying various ARGs including armA and blaCTX-M-15. The blaNDM-1 was located in a genetic context (IS26-∆ISAba125-blaNDM-1-ble-∆blaDHA-1-lysR-qacE∆1-ISCR1) identical (coverage, 100%; identity, 100%) to the one described for the IncL/M plasmid pNDM-OM from a clinical K. pneumoniae from Oman [24]. The blaCTX-M-15 was located on an IS26-flanked ~60kb IncR plasmid-derived sequence integrated into the ARI-A resistance island of the IncC plasmid. Except for the IncR plasmid-derived sequence, p3347689I_1 was highly similar (coverage, 98%; identity, 100%) to the IncC plasmid from E. coli 3347558 with armA located in an identical genetic context (Figure 1B). However, p3347689I_1 was missing the ISCR1-aph(3')-VI-ISAba125-blaNDM-1-∆ble-qacE∆1-sul1 region, suggesting an acquisition event in p3347558_1 (Figure 1B). Finally, we note that ST395 is a globally successful lineage that usually carries blaOXA-48 and blaKPC carbapenemase encoding-genes [25].
The CP P. stuartii strain 3347685 possessed blaOXA-48 and armA, consistent with the observed carbapenem and aminoglycoside phenotypic resistance (Table 2 and Table 3). We note that OXA-48-producing P. stuartii have been rarely reported and none of them co-produced ArmA or other 16S rRNA methylases leading to such pan-resistance to clinically used aminoglycosides.
In strain 3347685, blaOXA-48 was chromosome borne, and it was located within a Tn1999.2 transposon [26]. The armA was located on a 189kb IncC plasmid of type 1b (p3347685_1) in a genetic context identical to the one in the type 1a plasmids p3347558_1 from E. coli and p3347689I_1 from the CP K. pneumoniae (Figure 1B). However, in contrast to the latter 2 plasmids, p3347685_1 was missing the type 1a patch region and carried the ARI-B resistance island in addition to ARI-A [14].
The CP A. baumannii isolate was resistant to all ß-lactams including high-level resistance to carbapenems and carried the blaOXA-40 gene (Table 3). It was resistant to fluoroquinolones, but it remained susceptible to trimethoprim-sulfamethoxazole and to colistin (Table 2). It did not produce any additional ESBL or 16S rRNA methylase, remaining susceptible to tobramycin.
Citrobacter sedlakii: genomic and plasmid characterizations. Based on the WGS, the C. freundii complex strain 3347089 II was actually of C. sedlakii species. Moreover, the ANI values (≥98.98%) among our strain and the 6 C. sedlakii genome assemblies currently deposited in the NCBI genome database (accessed on 03/17/2021) confirmed the ID as C. sedlakii (data not shown). A core-genome analysis was performed including C. sedlakii 3347089 II, the 6 deposited genomes, and 3 assemblies deposited as Citrobacter spp. that were highly similar to 3347089 II based on the ANI values (>99%). As a result, no clonal relationship between the deposited assemblies and our C. sedlakii isolate could be observed (Supplemental Figure 1). It should be noted that only 2 blaNDM-positive C. sedlakii isolates were previously reported. However, the genomes of these 2 strains–respectively from Pakistan and Bangladesh–had not been sequenced [27, 28].
C. sedlakii 3347089 II carried a 167kb IncC type 1a plasmid and a 44kb IncR plasmid (Table 3). Remarkably, the IncC plasmid (p3347089II_1) was identical (identity, 100%) to the blaNDM-1 and armA carrying IncC plasmid from E. coli 3347558, except for the ∆ISAba14-aph(3')-VI-ISAba125 region that was missing (Figure 1B). Comparison of the 3 IncC type 1a plasmids from our study suggested a common ancestor with a sequence similar to the one of p3347689I_1 from K. pneumoniae, but missing the ISKpn21 and the IncR plasmid-derived insertion sequence. The ISCR1-flanked aph(3')-VI, blaNDM-1, and sul1 and the blaNDM-1 and sul1 in E. coli 3347558 and C. sedlakii 3347089 II, respectively, were likely acquired by a recombination event with the ISCR1 element (Figure 1B) [29].
An analysis of the NCBI deposited C. sedlakii genomes revealed that the Citrobacter spp. strain 50677481 (GenBank: GCA_001463265; Supplemental Figure 1) possessed blaNDM-1 and armA together with an IncC plasmid. Therefore, we further analyzed its Illumina-derived WGS assembly. Mapping of the contigs to the plasmid sequence pPS-T1 from the German P. stuartii allowed the reconstruction of the complete plasmid sequence and showed that Citrobacter spp. 50677481 harbored an IncC plasmid identical to p3347558_1 from our E. coli isolate (coverage, 100%; identity, 99.98%) (data not shown). Remarkably, the Citrobacter spp. strain 50677481 was isolated in 2012 from a Norwegian patient with travel history to Serbia, suggesting a persistent and wide distribution of this multidrug resistance plasmid in the Balkan region [30].
Finally, the 44kb IncR plasmid (p3347089II_2) carried by our C. sedlakii contained multiple MGEs and ARGs including the integrons In369 carrying aadA1 and dfrA1, and In1387 carrying aac(6')-Ib-cr, blaOXA-1 and catB3 (Table 3). The plasmid backbone of p3347089II_2 was identical (identity, 99.93%) to p12-6919.2, a 39kb IncR plasmid from a Salmonella enterica subsp. enterica isolate from Canada in 2012 (GenBank: CP039605).
Although reports of CPE from Macedonia are missing, studies from neighboring countries reported concerning levels of NDM producers [31–33]. Therefore, the risk of importing CPE through the transfer of patients from these countries to those with a low prevalence is concerning. This phenomenon has been extensively discussed before (eg, in [34–36]). The present study underlined the importance of monitoring such cases to prevent the importation of multiple difficult-to-treat pathogens carrying novel antibiotic resistance traits.
Furthermore, we noted that while OXA-40-producing A. baumannii strains have been extensively described [1], the 4 CPE carried by the patient presented unusual patterns of antimicrobial resistance. In fact, all CPE were co-resistant to all aminoglycosides due to the production of the ArmA 16S rRNA methylase [6]. More importantly, E. coli 3347558 and C. sedlakii 3347089 II carried the blaNDM-1 and armA ARGs in an identical IncC type 1a plasmid suggesting an in vivo conjugation event. We also noted that this IncC type 1a plasmid was identical to one found in Germany in a P. stuartii isolate and to another one carried by a Citrobacter spp. strain linked to Serbia [20, 30].
Overall, our findings emphasize the potential of the IncC plasmids carrying life-threatening ARGs to spread worldwide among different Enterobacterales. The presence of these broad-host-range MGEs in rare enterobacterial species (eg, C. sedlakii) should be further investigated to better comprehend their origin and future evolution.
Table 1. Summary of the samples and bacteria isolated from the patient during the routine tests
|
Daya |
Sample taken and results (if any)b |
||||
|
1 |
Three blood cultures: |
Rectal swab: |
Indwelling catheter tip: |
Nasal swab for MRSA: |
Wound swab (lower leg left): |
|
Swab at the insertion site of the venous catheter: |
Swab of a sacral ulcer: |
Swab at the insertion site of a permanent catheter: |
Swab of the tracheostomy tube wound: |
Wound swab (heel left): |
|
|
Swab of the left external malleolus: |
Swab of the right external malleolus: |
||||
|
2 |
Two blood cultures: |
Catheter tip: |
Urine (from permanent catheter): |
Wound biopsy (decubitus): |
Bone biopsy (sacrum): |
|
Wound biopsy (malleolus): |
Biopsy soft tissue (decubitus): |
Biopsy soft tissue (decubitus): |
|||
|
6 |
Two blood cultures: |
||||
|
12 |
Blood culture |
Wound swab (sacrum): |
|||
|
13 |
Two blood cultures: |
Central venous catheter (jugular): |
Arterial catheter (femoral): |
||
|
14e |
Tracheo- bronchial fluid: |
||||
a. Days from the hospitalization (admission at our institution in Bern, Switzerland)
b. Gram-negatives non-susceptible to carbapenems are reported in bold. “CP” indicates that these strains were carbapenemase producers according to the results of the Rapid Carba NP, CarbAcineto NP, NG-Test CARBA-5 and/or eazyplex assays implemented by the routine laboratory.
c. These bacteria were selected for WGS. We show their MIC values and genetic data in Table 2 and Table 3, respectively.
d. This strain was extended-spectrum cephalosporin-resistant (ESC-R), but carbapenem susceptible (see Table 2)
e. The patient was transferred to another Swiss institution
Table 2. Phenotypic characterization of the 6 Gram-negatives isolated from the same patient
|
Antibiotics |
Strain (species and lab code), sample and MICs (mg/L)a |
||||||||||||
|
E. colib 3347558 |
K. pneumoniaeb 3347684 II |
K. pneumoniaeb 3347689 I |
P. stuartiib 3347685 |
C. sedlakiib 3347689 II |
A. baumannii 3347684 I |
||||||||
|
Piperacillin-tazobactam |
>64 |
R |
<4 |
S |
>64 |
R |
>64 |
R |
>64 |
R |
>64 |
na |
|
|
Ticarcillin-clavulanate |
>128 |
R |
128 |
R |
>128 |
R |
>128 |
R |
>128 |
R |
>128 |
na |
|
|
Cefpodoxime |
>32 |
R |
>32 |
R |
>32 |
R |
>32 |
R |
>32 |
R |
nt |
||
|
Ceftazidime |
>128 |
R |
32 |
R |
>128 |
R |
128 |
R |
>128 |
R |
>128 |
na |
|
|
Ceftazidime- clavulanate |
>128 |
na |
0.25 |
na |
>128 |
na |
128 |
na |
>128 |
na |
nt |
||
|
Ceftriaxone |
>128 |
R |
128 |
R |
>128 |
R |
>128 |
R |
>128 |
R |
nt |
||
|
Cefotaxime |
>64 |
R |
64 |
R |
>64 |
R |
>64 |
R |
|
>64 |
R |
>64 |
na |
|
Cefotaxime- |
>64 |
na |
<0.125 |
na |
>64 |
na |
>64 |
na |
>64 |
na |
nt |
na |
|
|
Cefepime |
>16 |
R |
8 |
R |
>16 |
R |
>16 |
R |
>16 |
R |
16 |
na |
|
|
Aztreonam |
>16 |
R |
>16 |
R |
>16 |
R |
>16 |
R |
>16 |
R |
>16 |
na |
|
|
Imipenem |
1 |
S |
<0.5 |
S |
8 |
R |
2 |
I |
8 |
R |
>8 |
R |
|
|
Meropenem |
<1 |
S |
<1 |
S |
>8 |
R |
2 |
S |
8 |
R |
>8 |
R |
|
|
Doripenem |
0.5 |
na |
<0.125 |
na |
>2 |
na |
1 |
na |
>2 |
na |
>2 |
na |
|
|
Ertapenem |
2 |
R |
<0.25 |
S |
>4 |
R |
0.5 |
S |
>4 |
R |
>4 |
na |
|
|
Gentamicin |
>8 |
R |
>16 |
R |
>16 |
R |
>8 |
R |
>8 |
R |
>8 |
R |
|
|
Tobramycin |
>8 |
R |
>8 |
R |
>8 |
R |
>8 |
R |
>8 |
R |
4 |
S |
|
|
Amikacin |
>32 |
R |
<4 |
S |
>32 |
R |
>32 |
R |
>32 |
R |
>32 |
R |
|
|
Ciprofloxacin |
1 |
R |
2 |
R |
>2 |
R |
>2 |
R |
<0.25 |
S |
>2 |
R |
|
|
Levofloxacin |
<1 |
S |
<1 |
S |
8 |
R |
>8 |
R |
<1 |
S |
4 |
R |
|
|
Doxycycline |
>16 |
na |
<2 |
na |
16 |
na |
>16 |
na |
<2 |
na |
<2 |
na |
|
|
Minocycline |
8 |
na |
<2 |
na |
4 |
na |
>16 |
na |
<2 |
na |
<2 |
na |
|
|
Tigecycline |
<0.25 |
S |
0.5 |
na |
1 |
na |
2 |
na |
0.5 |
S |
<0.25 |
na |
|
|
Trimethoprim/sulfamethoxazole |
>4 |
R |
2 |
S |
>4 |
R |
4 |
I |
>4 |
R |
<0.5 |
S |
|
|
Colistin |
<0.25 |
S |
<0.25 |
S |
<0.25 |
S |
>4 |
na |
<0.25 |
S |
<0.25 |
S |
|
|
Polymyxin B |
0.5 |
na |
0.5 |
na |
0.5 |
na |
>4 |
na |
<0.25 |
na |
<0.25 |
na |
|
Note. R, resistant; I, susceptible, increased exposure; S, susceptible; na, not available or not applicable; nt, not tested a MICs were obtained with microdilution Sensititre panel GNX2F and ESB1F and interpreted according to the EUCAST 2019 criteria (version 9.0). A. baumannii was tested using only the GNX2F panel. b Species identification was obtained based on the WGS and implementing the hybrid WGS assembling
Table 3. Molecular characterization of the 5 Enterobacterales isolated from the same patient
|
Sequence ID |
GenBank |
Sequence type |
Length (bp) |
Inc group |
Antimicrobial resistance genes (ARGs)a |
Genetic environment of the main ARGsa |
|
E. coli, 3347558, ST69b, c |
||||||
|
3347558 |
CP071073 |
chromosome |
5'631'396 |
– |
mdf(A) |
|
|
p3347558_1 |
CP071074 |
plasmid |
169'082 |
C type 1a |
dfrA14, arr-2, cmlA1, |
ISAba125-blaNDM-1-ble ISEc28-armA-ISEc29 |
|
p3347558_2 |
CP071075 |
plasmid |
129'523 |
FIA, Y |
aph(3")-Ib, aph(6)-Id, blaTEM-1B, mph(A), tet(B), dfrA14, sul2 |
|
|
p3347558_3 |
CP071076 |
plasmid |
93'750 |
I1-I (Gamma) |
aac(3)-IId, blaCTX-M-15, blaTEM-1B |
IS26-∆ISEcp1-blaCTX-M-15-wbuC-IS26 |
|
p3347558_4 |
CP071077 |
plasmid |
64'917 |
FII |
nd |
|
|
p3347558_5 |
CP071078 |
plasmid |
5'167 |
nd |
nd |
|
|
p3347558_6 |
CP071079 |
plasmid |
4'072 |
nd |
nd |
|
|
K. pneumoniae, 3347684 II, ST268 b |
||||||
|
3347684 II |
CP071080 |
chromosome |
5'290'520 |
- |
blaSHV-11, oqxB, fosA5 |
|
|
p3347684 II_1 |
CP071081 |
plasmid |
155'851 |
FIB(K) |
nd |
|
|
p3347684 II_2 |
CP071082 |
plasmid |
110'998 |
FIB |
nd |
|
|
p3347684 II_3 |
CP071083 |
plasmid |
64'471 |
nd |
aac(3)-IIa, aac(6')-Ib-cr, blaOXA-1, blaCTX-M-15, qnrB1, catB3, dfrA14 |
ISEcp1-blaCTX-M-15-wbuC-Tn3 family tnp |
|
p3347684 II_4 |
CP071084 |
Plasmid |
63'577 |
FII(Yp) |
nd |
|
|
p3347684 II_5 |
CP071085 |
plasmid |
4'251 |
Col(pHAD28) |
nd |
|
|
K. pneumoniae, 3347689 I, ST395b |
||||||
|
3347689I |
CP071086 |
chromosome |
5'620'517 |
- |
aac(3)-IId, blaTEM-1B, blaNDM-1, blaSHV-182, oqxA, oqxB, sul1, fosA |
IS26-∆ISAba125-blaNDM-1-ble-∆blaDHA-1-lysR-qacE∆1-ISCR1 |
|
p3347689I_1 |
CP071087 |
plasmid |
222'786 |
C type 1a / R |
dfrA14, arr-2, cmlA1, blaOXA-10, aadA1, sul1, aph(3')-VI, armA, msr(E), mph(E), blaCMY-4, aac(3)-IIa, aac(6')-Ib-cr, blaOXA-1, blaCTX-M-15, tet(A), tet(R), catA1, dfrA1 |
ISEc28-armA-ISEc29 IS26-ORF-wbuC-blaCTX-M-15-IS26-Tn3 family tnp |
|
p3347689I_2 |
CP071088 |
plasmid |
9'730 |
ColRNAI |
Nd |
|
|
p3347689I_3 |
CP071089 |
plasmid |
4'052 |
Col440II |
Nd |
|
|
P. stuartii, 3347685b |
||||||
|
3347685 |
CP071068 |
chromosome |
4'476'038 |
- |
aac(2')-Ia, blaCTX-M-15, blaOXA-48, tet(B), catA3, dfrA14 |
IS1999-IS1R-blaOXA-48-lysR-IS1999 |
|
p3347685_1 |
CP071069 |
plasmid |
188'750 |
C type 1b |
dfrA14, arr-2, cmlA1, blaOXA-10, aadA1, sul1, armA, msr(E), mph(E), blaCMY-4, aac(6')Ib-cr, blaOXA-1, blaCTX-M-15, tet(A), aadA2, aph(3")-Ib, aph(6)-Id, blaTEM-1B, dfrA12, sul2 |
ISEc28-armA-ISEc29 |
|
C. sedlakii, 3347689 IIb |
||||||
|
3347689 II |
CP071070 |
chromosome |
4'756'279 |
- |
blaSED-1 |
|
|
p3347689 II_1 |
CP071071 |
plasmid |
166'860 |
C type 1a |
dfrA14, arr-2, cmlA1, blaOXA-10, aadA1, sul1, blaNDM-1, armA, msr(E), mph(E), blaCMY-4 |
ISAba125-blaNDM-1-bleMBL ISEc28-armA-ISEc29 |
|
p3347689 II_2 |
CP071072 |
plasmid |
44'080 |
R |
aadA1, dfrA1, aac(6')-Ib-cr, blaOXA-1, catB3, sul1, mph(A), blaSHV-12 |
|
Note. nd, none detecteda The main ARGs are in bold b All sequences were obtained by a hybrid WGS sequencing approach combining Illumina and Nanopore reads c The upstream region of the chromosomal AmpC did not contain mutations able to improve the expression of the bla gene
The authors report no relevant conflicts of interest to disclose.
Supplementary materials are available at the Pathogens and Immunity website. Supplementary data may be provided by the authors to benefit the reader. Supplementary data are not copyedited and are the sole responsibility of the authors. Questions or comments related to supplementary materials should be addressed to the corresponding author.
This work was supported by NRP-72, “National Research Programme, Antimicrobial Resistance” (Swiss National Science Foundation, SNF; grant No. 177378 to AE), by SNF grant 192514 (to AE), and by the University of Fribourg by the Swiss National Reference center for Emerging Antibiotic Resistance (NARA).
1. Nordmann P, Poirel L. Epidemiology and Diagnostics of Carbapenem Resistance in Gram-negative Bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521-S8. doi: 10.1093/cid/ciz824. PubMed PMID: 31724045; PMCID: PMC6853758.
2. Bonomo RA, Burd EM, Conly J, Limbago BM, Poirel L, Segre JA, Westblade LF. Carbapenemase-Producing Organisms: A Global Scourge. Clin Infect Dis. 2018;66(8):1290-7. doi: 10.1093/cid/cix893. PubMed PMID: 29165604; PMCID: PMC5884739.
3. Bush K. Past and Present Perspectives on beta-Lactamases. Antimicrob Agents Chemother. 2018;62(10). doi: 10.1128/AAC.01076-18. PubMed PMID: 30061284; PMCID: PMC6153792.
4. Dortet L, Poirel L, Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int. 2014;2014:249856. doi: 10.1155/2014/249856. PubMed PMID: 24790993; PMCID: PMC3984790.
5. Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. NDM Metallo-beta-Lactamases and Their Bacterial Producers in Health Care Settings. Clin Microbiol Rev. 2019;32(2). doi: 10.1128/CMR.00115-18. PubMed PMID: 30700432; PMCID: PMC6431124.
6. Wachino JI, Doi Y, Arakawa Y. Aminoglycoside Resistance: Updates with a Focus on Acquired 16S Ribosomal RNA Methyltransferases. Infect Dis Clin North Am. 2020;34(4):887-902. doi: 10.1016/j.idc.2020.06.002. PubMed PMID: 33011054.
7. Tijet N, Muller MP, Matukas LM, Khan A, Patel SN, Melano RG. Lateral dissemination and inter-patient transmission of blaKPC-3: role of a conjugative plasmid in spreading carbapenem resistance. J Antimicrob Chemother. 2016;71(2):344-7. doi: 10.1093/jac/dkv356. PubMed PMID: 26518052.
8. Gona F, Barbera F, Pasquariello AC, Grossi P, Gridelli B, Mezzatesta ML, Caio C, Stefani S, Conaldi PG. In vivo multiclonal transfer of bla(KPC-3) from Klebsiella pneumoniae to Escherichia coli in surgery patients. Clin Microbiol Infect. 2014;20(10):O633-5. doi: 10.1111/1469-0691.12577. PubMed PMID: 24476498.
9. Aires-de-Sousa M, Ortiz de la Rosa JM, Goncalves ML, Costa A, Nordmann P, Poirel L. Occurrence of NDM-1-producing Morganella morganii and Proteus mirabilis in a single patient in Portugal: probable in vivo transfer by conjugation. J Antimicrob Chemother. 2020;75(4):903-6. doi: 10.1093/jac/dkz542. PubMed PMID: 31971235.
10. Arana DM, Saez D, Garcia-Hierro P, Bautista V, Fernandez-Romero S, Angel de la Cal M, Alos JI, Oteo J. Concurrent interspecies and clonal dissemination of OXA-48 carbapenemase. Clin Microbiol Infect. 2015;21(2):148 e1-4. doi: 10.1016/j.cmi.2014.07.008. PubMed PMID: 25596781.
11. Hammerum AM, Hansen F, Nielsen HL, Jakobsen L, Stegger M, Andersen PS, Jensen P, Nielsen TK, Hansen LH, Hasman H, Fuglsang-Damgaard D. Use of WGS data for investigation of a long-term NDM-1-producing Citrobacter freundii outbreak and secondary in vivo spread of blaNDM-1 to Escherichia coli, Klebsiella pneumoniae and Klebsiella oxytoca. J Antimicrob Chemother. 2016;71(11):3117-24. doi: 10.1093/jac/dkw289. PubMed PMID: 27494919.
12. Zhu B, Ying C, Xu H, Ying J. Coexistence of NDM-1-producing Escherichia coli and Citrobacter freundii in the same patient. J Glob Antimicrob Resist. 2018;15:79-81. doi: 10.1016/j.jgar.2018.04.013. PubMed PMID: 29727717.
13. Bosch T, Lutgens SPM, Hermans MHA, Wever PC, Schneeberger PM, Renders NHM, Leenders A, Kluytmans J, Schoffelen A, Notermans D, Witteveen S, Bathoorn E, Schouls LM. Outbreak of NDM-1-Producing Klebsiella pneumoniae in a Dutch Hospital, with Interspecies Transfer of the Resistance Plasmid and Unexpected Occurrence in Unrelated Health Care Centers. J Clin Microbiol. 2017;55(8):2380-90. doi: 10.1128/JCM.00535-17. PubMed PMID: 28515215; PMCID: PMC5527415.
14. Ambrose SJ, Harmer CJ, Hall RM. Evolution and typing of IncC plasmids contributing to antibiotic resistance in Gram-negative bacteria. Plasmid. 2018;99:40-55. doi: 10.1016/j.plasmid.2018.08.001. PubMed PMID: 30081066.
15. Endimiani A, Ramette A, Rhoads DD, Jacobs MR. The Evolving Role of the Clinical Microbiology Laboratory in Identifying Resistance in Gram-Negative Bacteria: An Update. Infect Dis Clin North Am. 2020;34(4):659-76. doi: 10.1016/j.idc.2020.08.001. PubMed PMID: 33011047.
16. Bonnin RA, Nordmann P, Poirel L. Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art. Expert Rev Anti Infect Ther. 2013;11(6):571-83. doi: 10.1586/eri.13.38. PubMed PMID: 23750729.
17. Campos-Madueno EI, Bernasconi OJ, Moser AI, Keller PM, Luzzaro F, Maffioli C, Bodmer T, Kronenberg A, Endimiani A. Rapid Increase of CTX-M-Producing Shigella sonnei Isolates in Switzerland Due to Spread of Common Plasmids and International Clones. Antimicrob Agents Chemother. 2020;64(10). doi: 10.1128/AAC.01057-20. PubMed PMID: 32718957; PMCID: PMC7508577.
18. Campos-Madueno EI, Gmuer C, Risch M, Bodmer T, Endimiani A. Characterisation of a new blaVIM-1-carrying IncN2 plasmid from an Enterobacter hormaechei subsp. steigerwaltii. J Glob Antimicrob Resist. 2021;24:325-7. doi: 10.1016/j.jgar.2021.01.017. PubMed PMID: 33571706.
19. Dadashi M, Yaslianifard S, Hajikhani B, Kabir K, Owlia P, Goudarzi M, Hakemivala M, Darban-Sarokhalil D. Frequency distribution, genotypes and prevalent sequence types of New Delhi metallo-beta-lactamase-producing Escherichia coli among clinical isolates around the world: A review. J Glob Antimicrob Resist. 2019;19:284-93. doi: 10.1016/j.jgar.2019.06.008. PubMed PMID: 31212107.
20. Weber RE, Pietsch M, Fruhauf A, Pfeifer Y, Martin M, Luft D, Gatermann S, Pfennigwerth N, Kaase M, Werner G, Fuchs S. IS26-Mediated Transfer of bla NDM-1 as the Main Route of Resistance Transmission During a Polyclonal, Multispecies Outbreak in a German Hospital. Front Microbiol. 2019;10:2817. doi: 10.3389/fmicb.2019.02817. PubMed PMID: 31921015; PMCID: PMC6929489.
21. Jovcic B, Lepsanovic Z, Begovic J, Rakonjac B, Perovanovic J, Topisirovic L, Kojic M. The clinical isolate Pseudomonas aeruginosa MMA83 carries two copies of the blaNDM-1 gene in a novel genetic context. Antimicrob Agents Chemother. 2013;57(7):3405-7. doi: 10.1128/AAC.02312-12. PubMed PMID: 23612199; PMCID: PMC3697382.
22. Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56(2):1087-9. doi: 10.1128/AAC.05620-11. PubMed PMID: 22143526; PMCID: PMC3264265.
23. Bercot B, Poirel L, Nordmann P. Plasmid-mediated 16S rRNA methylases among extended-spectrum beta-lactamase-producing Enterobacteriaceae isolates. Antimicrob Agents Chemother. 2008;52(12):4526-7. doi: 10.1128/AAC.00882-08. PubMed PMID: 18838598; PMCID: PMC2592896.
24. Bonnin RA, Nordmann P, Carattoli A, Poirel L. Comparative genomics of IncL/M-type plasmids: evolution by acquisition of resistance genes and insertion sequences. Antimicrob Agents Chemother. 2013;57(1):674-6. doi: 10.1128/AAC.01086-12. PubMed PMID: 23114767; PMCID: PMC3535931.
25. Di Pilato V, Errico G, Monaco M, Giani T, Del Grosso M, Antonelli A, David S, Lindh E, Camilli R, Aanensen DM, Rossolini GM, Pantosti A, pneumoniae A-ILSGoc-pK. The changing epidemiology of carbapenemase-producing Klebsiella pneumoniae in Italy: toward polyclonal evolution with emergence of high-risk lineages. J Antimicrob Chemother. 2021;76(2):355-61. doi: 10.1093/jac/dkaa431. PubMed PMID: 33188415.
26. Pitout JDD, Peirano G, Kock MM, Strydom KA, Matsumura Y. The Global Ascendency of OXA-48-Type Carbapenemases. Clin Microbiol Rev. 2019;33(1). doi: 10.1128/CMR.00102-19. PubMed PMID: 31722889; PMCID: PMC6860007.
27. Qamar MU, Walsh TR, Toleman MA, Tyrrell JM, Saleem S, Aboklaish A, Jahan S. Dissemination of genetically diverse NDM-1, -5, -7 producing-Gram-negative pathogens isolated from pediatric patients in Pakistan. Future Microbiol. 2019;14:691-704. doi: 10.2217/fmb-2019-0012. PubMed PMID: 31148474.
28. Rakhi NN, Alam A, Sultana M, Rahaman MM, Hossain MA. Diversity of carbapenemases in clinical isolates: The emergence of blaVIM-5 in Bangladesh. J Infect Chemother. 2019;25(6):444-51. doi: 10.1016/j.jiac.2019.01.010. PubMed PMID: 30824303.
29. Toleman MA, Bennett PM, Walsh TR. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol Mol Biol Rev. 2006;70(2):296-316. doi: 10.1128/MMBR.00048-05. PubMed PMID: 16760305; PMCID: PMC1489542.
30. Samuelsen O, Overballe-Petersen S, Bjornholt JV, Brisse S, Doumith M, Woodford N, Hopkins KL, Aasnaes B, Haldorsen B, Sundsfjord A, Norwegian Study Group on CPE. Molecular and epidemiological characterization of carbapenemase-producing Enterobacteriaceae in Norway, 2007 to 2014. PLoS One. 2017;12(11):e0187832. doi: 10.1371/journal.pone.0187832. PubMed PMID: 29141051; PMCID: PMC5687771.
31. Savov E, Politi L, Spanakis N, Trifonova A, Kioseva E, Tsakris A. NDM-1 Hazard in the Balkan States: Evidence of the First Outbreak of NDM-1-Producing Klebsiella pneumoniae in Bulgaria. Microb Drug Resist. 2018;24(3):253-9. doi: 10.1089/mdr.2017.0230. PubMed PMID: 28876169.
32. Brkic S, Bozic D, Stojanovic N, Vitorovic T, Topalov D, Jovanovic M, Stepanovic M, Cirkovic I. Antimicrobial Susceptibility and Molecular Characterization of Carbapenemase-Producing Enterobacter spp. Community Isolates in Belgrade, Serbia. Microb Drug Resist. 2020;26(4):378-84. doi: 10.1089/mdr.2019.0224. PubMed PMID: 31651210.
33. Politi L, Gartzonika K, Spanakis N, Zarkotou O, Poulou A, Skoura L, Vrioni G, Tsakris A. Emergence of NDM-1-producing Klebsiella pneumoniae in Greece: evidence of a widespread clonal outbreak. J Antimicrob Chemother. 2019;74(8):2197-202. doi: 10.1093/jac/dkz176. PubMed PMID: 31065697.
34. Seiffert SN, Perreten V, Johannes S, Droz S, Bodmer T, Endimiani A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob Agents Chemother. 2014;58(4):2446-9. doi: 10.1128/AAC.02417-13. PubMed PMID: 24468781; PMCID: PMC4023741.
35. Seiffert SN, Marschall J, Perreten V, Carattoli A, Furrer H, Endimiani A. Emergence of Klebsiella pneumoniae co-producing NDM-1, OXA-48, CTX-M-15, CMY-16, QnrA and ArmA in Switzerland. Int J Antimicrob Agents. 2014;44(3):260-2. doi: 10.1016/j.ijantimicag.2014.05.008. PubMed PMID: 25123809.
36. Clement M, Keller PM, Bernasconi OJ, Stirnimann G, Frey PM, Bloemberg GV, Sendi P, Endimiani A. First Clinical Case of In Vivo Acquisition of DHA-1 Plasmid-Mediated AmpC in a Salmonella enterica subsp. enterica Isolate. Antimicrob Agents Chemother. 2019;63(10). doi: 10.1128/AAC.00992-19. PubMed PMID: 31358582; PMCID: PMC6761535.
Submitted September 8, 2021 | Accepted October 11, 2021 | Published November 22, 2021
Copyright © 2021 Pathogens and Immunity. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.