Jessica Kumar1*, Brandon Eilertson2*, Jennifer L. Cadnum3, Chauna S. Whitlow4, Annette L. Jencson3, Nasia Safdar5,6, Sarah L. Krein7, Windy D. Tanner8, JeanMarie Mayer8, Matthew H. Samore8, Curtis J. Donskey1,9
1
Geriatric Research, Education, and Clinical Center; Louis Stokes Cleveland
VA Medical; Cleveland, Ohio
2
SUNY Downstate Medical Center; Brooklyn, New York
3
Research Service; Louis Stokes Cleveland VA Medical Center; Cleveland, Ohio
4
Pathology and Laboratory Medicine Service; Louis Stokes Cleveland VA
Medical Center; Cleveland, Ohio
5
Infectious Disease Division; University of Wisconsin-Madison School of
Medicine and Public Health; Madison, Wisconsin
6
William S. Middleton Memorial Veterans Hospital; Madison, Wisconsin
7
VA Ann Arbor Healthcare System; Ann Arbor, Michigan
8
University of Utah School of Medicine; Division of Epidemiology; Salt Lake
City, Utah
9
Case Western Reserve University School of Medicine; Cleveland, Ohio
* These authors contributed equally.
Curtis J. Donskey
Geriatric Research, Education and Clinical Center
Louis Stokes Cleveland VA Medical Center
10701 East Boulevard, Cleveland, Ohio 44106
Phone: 216-791-3800 x4788
Fax: 216-229-2403
Curtis.Donskey@va.gov
Background: Environmental sources have been implicated as a potential source for exogenous acquisition of Candida species, particularly the emerging multidrug-resistant Candida auris. However, limited information is available on environmental reservoirs of Candida species in healthcare facilities.
Methods: During a 6-month period, cultures for Candida species were collected from high-touch surfaces in patient rooms and from portable equipment in 6 US acute care hospitals in 4 states. Additional cultures were collected from sink drains and floors in one of the hospitals and from high-touch surfaces, portable equipment, and sink drains in a hospital experiencing an outbreak due to C. auris. Candida species were identified using matrix-assisted laser desorption/ionization time-of-flight mass spectometry.
Results: Candida species were recovered from patient rooms in 4 of the 6 hospitals. Seven of 147 patient room cultures (4.8%) and 1 of 57 (1.8%) portable equipment cultures were positive, with the most common species being C. parapsilosis. For the hospital where additional sites were sampled, Candida species were recovered from 8 of 22 (36.4%) hospital room floors and 4 of 17 (23.5%) sink drains. In the facility with a C. auris outbreak, Candida species were frequently recovered from sink drains (20.7%) and high-touch surfaces (15.4%), but recovery of C. auris was uncommon (3.8% of high-touch surfaces, 3.4% of sink drains, and 0% of portable equipment) and only present in rooms that currently or recently housed a patient with C. auris.
Conclusion: Candida species often contaminate surfaces in hospitals and may be particularly common on floors and in sink drains. However, C. auris contamination was uncommon in a facility experiencing an outbreak, suggesting that current cleaning and disinfection practices can be effective in minimizing environmental contamination.
Keywords: Candida auris; Candida species; environmental contamination; infection control; floor and sink drain disinfection
Candida species are an important cause of invasive infections in healthcare settings, particularly in immunocompromised and critically ill patients [1, 2]. Although most infections due to Candida species are endogenous, exogenous acquisition may also occur in healthcare facilities [1, 3-14]. In a recent study of the molecular epidemiology of candidemia in Iceland, molecular typing with polymerase chain reaction fingerprinting suggested that 19% to 40% of all infections were part of nosocomial clusters not identified by routine hospital surveillance [12]. The hands of healthcare personnel are considered the most important source of patient-to-patient transmission of Candida species [1, 4, 7]. Hand contamination with healthcare-associated pathogens may be acquired through direct contact with colonized or infected patients or after contact with contaminated surfaces [15, 16]. Some studies have implicated environmental sources, including contaminated portable equipment, as a source for acquisition of Candida species. Sanchez et al [4] demonstrated that a Candida parapsilosis strain causing infections was recovered from inanimate surfaces in a new intensive care unit before patients were admitted. The emerging fungal pathogen Candida auris has frequently been recovered from environmental surfaces in rooms of colonized or infected patients [13], and a recent report of an outbreak of C. auris in an intensive care unit linked transmission to shared temperature probes [14]. The ability of C. auris, C. parapsilosis, and Candida glabrata strains to survive for prolonged periods on dry and moist surfaces may increase the likelihood of transmission from the environment [17].
Given the importance of Candida species as healthcare-associated pathogens, there is a need for additional studies of potential environmental reservoirs. In a single hospital, we previously reported that Candida species were recovered frequently from the environment, particularly from moist surfaces [17]. Moreover, we found that Candida species exhibited relative resistance to widely used quaternary ammonium disinfectants and ultraviolet light [18, 19]. Here, we conducted a culture survey in 6 hospitals to assess the frequency of recovery of Candida species from high-touch surfaces in patient rooms. To obtain information on contamination of sites such as floors and sink drains, we conducted additional culture surveys in 2 hospitals, including a facility experiencing a C. auris outbreak.
The institutional review boards of the 6 facilities participating in the multihospital survey approved the study protocol. For the facility experiencing the Candida auris outbreak, collection of environmental cultures was conducted as a quality improvement project to assess the efficacy of cleaning and disinfection practices. No information that could identify patients was collected.
During a 6-month period from April 2016 to June 2017, environmental cultures for multiple healthcare-associated pathogens were collected in 6 US acute care hospitals in 4 states. The facilities used sporicidal disinfectants for high-touch surfaces in rooms of patients with Clostridioides difficile infection (CDI), non-sporicidal disinfectants for other patient rooms, and detergents for floors. The facilities included 2 tertiary care medical centers and 4 Veterans Affairs Medical Centers. A detailed description of the study and the results of bacterial cultures will be presented elsewhere. Samples from occupied contact precautions rooms and non-isolation rooms were cultured on multiple general medical/surgical wards or intensive care units, with culture collections occurring at each facility every 2 weeks for 8 to 11 cycles. Up to 4 contact precautions rooms (depending on availability) and an equal number of non-isolation rooms were sampled during each culture collection.
For each patient room, composite cultures were collected from standardized sites < 3 feet from the patient (bed rail, bedside table, telephone, and call button), > 3 feet from the patient inside the room (room inner door handle, charting bar code scanner and keyboard, and the bed control panel), and in the bathroom (toilet grab bar, toilet flush handle, toilet rinse spout handle, and inner bathroom door handle). If the room occupant was using a commode, the grab handles and commode seat were sampled in place of the bathroom samples. Standardized surfaces from shared equipment were also sampled in each unit. The shared unit equipment sample was a composite of surfaces from a glucometer, bladder scanner probe handle, and Doppler ultrasound or portable vital signs monitor.
All cultures were collected using cellulose sponges (Sponge Stick with neutralizing buffer, 3M). The entire surface area of each culture site was contacted with the Sponge Stick which was pre-moistened with neutralizing buffer. The specimens were processed as previously described [20-22]. After completion of bacterial cultures, the specimens were frozen at -80ºC in 15% glycerol as a cryoprotectant and thawed for Candida species cultures; 500 µL of each sample was plated onto Sabouraud dextrose agar and incubated for 96 hours at 37ºC. Colonies consistent with Candida species were subjected to identification using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Bruker Biotyper CA System, Bellerica, MA). Preliminary studies indicated that freezing of the specimens did not significantly reduce the yield of Candida species (data not shown).
A recent study demonstrated that sink drains may be a potential source for dispersal of Candida species [22]. It has also been suggested that hospital floors might be an underappreciated source for dissemination of healthcare-associated pathogens [23, 24]. Therefore, we collected an additional set of cultures in one of the study hospitals to obtain information on contamination of sink drains and floors. The facility was a Veterans Affairs Medical Center. The facility used a sporicidal disinfectant for high-touch surfaces in rooms of patients with CDI, a non-sporicidal disinfectant for other patient rooms, and a detergent for floors. Sink bowls were routinely disinfected during post-discharge room cleaning and disinfection, but no sink drain disinfection was performed. The cultures were collected using BBL Culture Swabs (Becton Dickinson, Cockeysville, MD). For floors, pre-moistened swabs were used to sample a total area of 20 × 20 cm from sites adjacent to the bed, adjacent to the trash can, and in the bathroom. For sink drains, the swabs were inserted through the strainer and samples were collected from the surface of the drain pipes to a depth of 1 inch below the strainer. For comparison, a composite of the high-touch surfaces < 3 feet from the patient (described previously) was sampled with a single pre-moistened swab. The swabs were plated directly onto Sabouraud dextrose agar. The cultures were processed as described previously and colonies consistent with Candida species were identified using MALDI-TOF.
We collected environmental cultures in a 376-bed tertiary care hospital located in Brooklyn, New York which was experiencing a C. auris outbreak when the cultures were collected. The hospital experiencing the C. auris outbreak provided care for a total of 8 patients with C. auris, including 3 with positive clinical cultures (1 with candidemia and 2 with positive urine cultures that were deemed asymptomatic funguria) and 5 with colonization identified by culturing samples from the groin and axilla. The first patient was admitted in September, 2017, but carriage of C. auris was not recognized until November, 2017. A second patient was admitted in January, 2018 and developed candidemia in February, 2018. In May-June, 2018, 6 colonized patients were detected through screening. The final identified patient with C. auris was discharged during the last week of June, 2018.
An initial set of environmental cultures was obtained in May, 2018. During this period, routine cleaning and disinfection of patient rooms included use of a quaternary ammonium disinfectant once daily for disinfection of high-touch surfaces and a bleach wipe product for post-discharge cleaning and disinfection. In C. auris rooms, bleach wipes were used for once-daily disinfection of high-touch surfaces and floors were cleaned with a detergent; sink bowls were disinfected during post-discharge room cleaning and disinfection, but no sink drain disinfection was performed. At the time the initial cultures were collected, 3 patients colonized with C. auris were in the facility, including 2 in a 2-bed room. The rooms of the C. auris patients and other rooms that had previously housed C. auris patients were included in the culture survey. A second set of cultures was obtained in June, 2018 after switching to a chlorine-based spray disinfectant for routine cleaning and disinfection of all patient rooms; the frequency of daily disinfection in C. auris rooms was also increased to 3-times daily. Three patients colonized with C. auris were in the facility at the time of the second set of cultures, and their rooms were included in the culture survey.
The cultures were collected using BBL Culture Swabs as described previously. For sink drains, the swabs were inserted through the strainer and samples were collected from the surface of the drain pipes to a depth of 1 inch below the strainer. For high-touch surfaces and portable equipment, 5 × 10 cm areas of larger surfaces were sampled and the entire surface area of smaller items (eg, call buttons, telephones) was sampled. The swabs were plated directly onto Sabouraud dextrose agar. The cultures were processed as described previously and colonies consistent with Candida species were identified using MALDI-TOF.
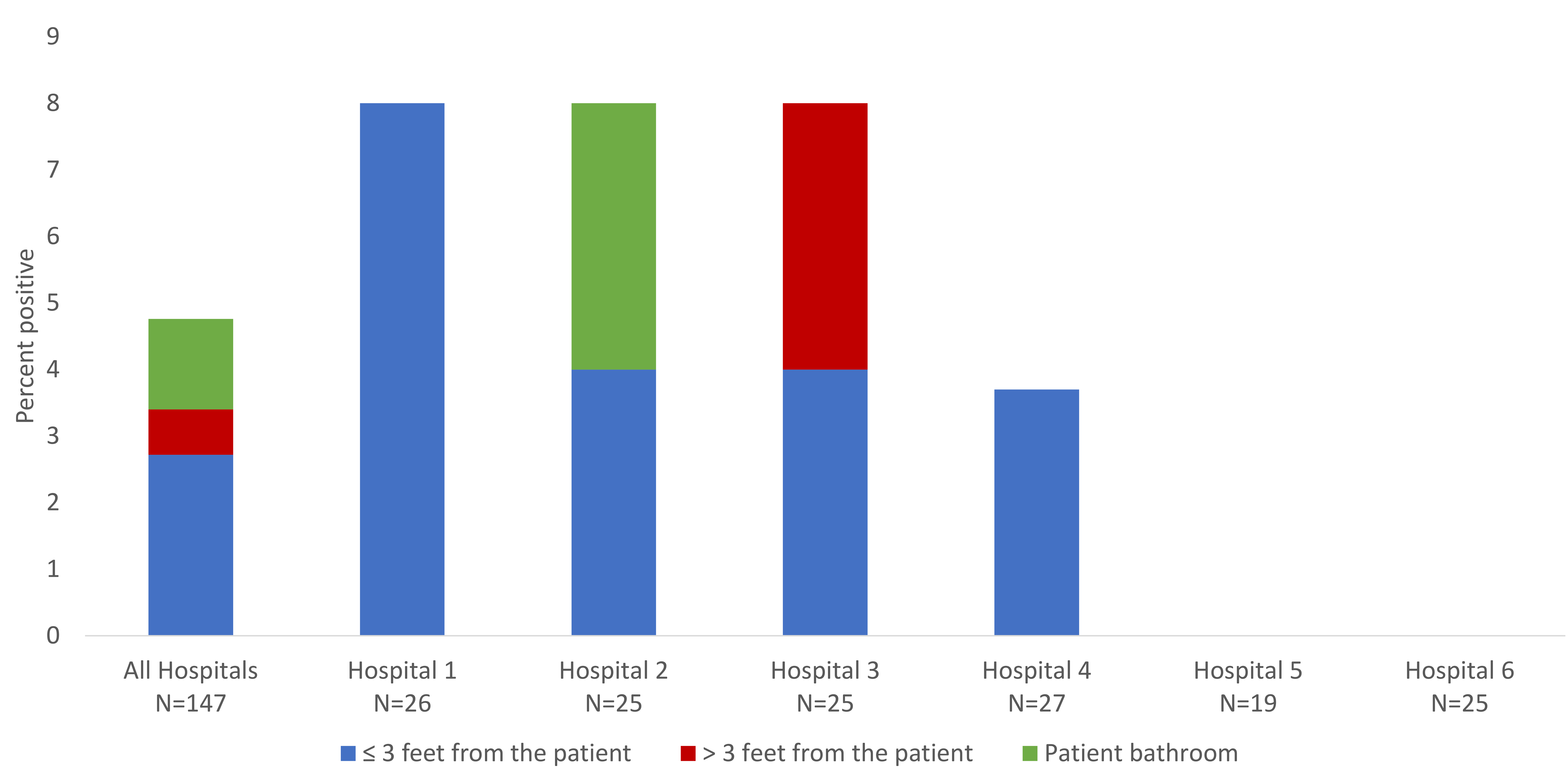
Figure 1 shows the percentage of patient rooms with positive cultures for Candida species from high-touch surfaces in the 6 study hospitals and the sites of recovery. Candida species were recovered from 1 or more rooms in 4 of the 6 hospitals. Overall, Candida species were recovered from 7 of 147 rooms (4.8%); 8 total sites were positive (ie, 1 contaminated room was positive both <3 feet and > 3 feet from the patient). The Candida species recovered included Candida parapsilosis (N = 5), Candida lusitaniae (N = 3), and Candida guilliermondii (N = 1). Contamination was detected in 4 (3.1%) rooms < 3 feet from the patient, 1 (0.7%) room > 3 feet from the patient, and 2 (1.4%) bathrooms. The frequency of recovery of Candida species was similar for contact precautions rooms and non-isolation rooms (3 of 67, 4.5% versus 4 of 80, 5.0%). Candida species were recovered from 1 of 57 (1.8%) sets of cultures of portable equipment cultured in the 6 hospitals.

Figure 1. Frequency of recovery of Candida species from environmental surfaces in patient rooms in 6 hospitals.
Additional cultures were collected from the hospital labeled hospital 1 in Figure 1. Candida species were recovered from 8 of 22 (36.4%) hospital room floors, 4 of 17 (23.5%) sink drains, and 2 of 22 (9.1%) high-touch surfaces. The species recovered from floors included C. parapsilosis, C. metapsilosis, C. orthopsilosis, C. glabrata, and C. albicans. The species recovered from sink drains included C. parapsilosis and C. tropicalis. The species recovered from high-touch surfaces included C. parapsilosis, C. lusitaniae, and C. guillermondii. The number of colony-forming units of Candida species recovered from sink drains (mean = 90, range 8 to 300 CFU) was higher than the number recovered from floors or high-touch surfaces (mean = 2, range 1 to 4 CFU).
Table 1 shows the results of cultures collected from the hospital experiencing the C. auris outbreak. The percentages of cultures positive for Candida species were similar for cultures collected during the disinfectant protocols 1 and 2. However, C. auris was only recovered from high-touch surfaces during disinfectant protocol 1 when a quaternary ammonium disinfectant was used once-daily in non-C. auris rooms and bleach wipes were used once-daily and post-discharge in C. auris rooms. One sink drain was positive for C. auris during disinfectant protocol 2 when a chlorine-based disinfectant was used for routine cleaning and disinfection of all patient rooms and 3-times daily and post-discharge in C. auris rooms. No portable equipment cultures were positive for C. auris. The high-touch surfaces with positive cultures for C. auris included a bedrail/bedside table and a call button/telephone.
Table 1. Recovery of Candida species from environmental cultures in an acute care hospital experiencing an outbreak of Candida auris during periods when different disinfectant protocols were used.
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*, Disinfectant Protocol 1: routine cleaning and disinfection of patient rooms included use of a quaternary ammonium disinfectant once daily for disinfection of high-touch surfaces and a bleach wipe product for post-discharge cleaning and disinfection; in C. auris rooms bleach wipes were used for once-daily disinfection of high-touch surfaces.
**, Disinfectant Protocol 2: routine cleaning and disinfection of patient rooms included use of a chlorine-based spray disinfectant for routine cleaning and disinfection of all patient rooms; the frequency of daily disinfection in C. auris rooms was increased to 3-times daily.
Although many studies have investigated contamination of the healthcare environment with bacterial pathogens, little information is available on the frequency of contamination with Candida species. In 6 hospitals, we recovered Candida species from high-touch surfaces in 4.8% of patient rooms and from 1.8% of shared portable equipment. In one of the hospitals, additional cultures demonstrated frequent recovery of Candida species from sink drains (23.5%) and floors in patient rooms (36.4%). It is likely that more frequent contamination may have been detected on high-touch surfaces if rooms of patients with known Candida species colonization or infection had been sampled.
In a hospital experiencing an outbreak of C. auris, Candida species were frequently recovered from sink drains (20.7%) and high-touch surfaces (15.4%). However, C. auris was rarely recovered (3.8% of high-touch surfaces, 3.4% of sink drains, and 0% of portable equipment) and only from sites in rooms that currently or recently housed a patient colonized or infected with C. auris. It is notable that C. auris was recovered from high-touch surfaces during the period when once-daily bleach wipe disinfection was performed in C. auris rooms, but not when the frequency of disinfection was increased to 3-times daily with a chlorine-based spray disinfectant. Although the conclusions that can be drawn from these findings are limited, the data suggest that current cleaning practices including frequent application of a sporicidal disinfectant in C. auris rooms can be effective in minimizing environmental contamination. The finding of a positive sink drain culture during the second point-prevalence culture survey does raise some concern of the potential for sink drains to be a reservoir for C. auris persistence which is not amenable to cleaning and disinfection. It has been demonstrated that Candida species colonizing sink drains may disperse to countertops by splashing of water during sink operation [22].
We found that Candida species contamination was more common on high-touch surfaces in close proximity to patients (3.1% contamination on surfaces < 3 feet from the patient and 0.7% on surfaces > 3 feet from the patient). Surfaces in close proximity to patients may be more frequently contaminated than more distant surfaces in patient rooms [25, 26]. In addition, it has been demonstrated that during procedures shedding of methicillin-resistant Staphylococcus aureus (MRSA) by colonized patients occurs significantly more often on surfaces less than 3 feet versus greater than 3 feet from the patient [27]. Therefore, it has been proposed that personnel remaining in more distant lower-risk areas of contact precautions rooms might not be required to wear personal protective equipment if they do not contact surfaces [25, 26].
One striking finding from our study was the high frequency of floor contamination with Candida species in the single hospital where floors were sampled. This finding is consistent with previous studies that have demonstrated that the floor is typically the most heavily contaminated environmental site in healthcare facilities [28]. The significance of floor contamination is uncertain. However, it has been suggested that hospital floors might be an underestimated source for dissemination of healthcare-associated pathogens because organisms on floors can be transferred from shoes or socks or other high-touch items to the hands of patients or personnel [23, 24, 28].
Our study has some limitations. We did not have information on whether the patients in the study rooms were colonized or infected with Candida species. Studies are needed to assess environmental contamination in rooms known to be occupied by colonized or infected patients, including on wards with patients at high risk for Candida species infection (eg, oncology wards). Our study does not provide information on whether environmental sites are contaminated through direct contact with colonized patients or via the hands of personnel. In addition, we did not confirm that Candida species in the environment can be transferred to patients or acquired on the hands of personnel. Previous studies have demonstrated that C. difficile and MRSA contamination on surfaces is frequently acquired on hands [15, 16]. Future studies are needed to determine if environmental isolates of Candida species are molecularly related to isolates that colonize or infect patients or are carried on the hands of personnel. Finally, floor cultures were collected in only a single hospital. Additional studies are needed to evaluate floor contamination with Candida species, particularly in facilities with C. auris outbreaks.
Our findings demonstrate that Candida species often contaminate surfaces in hospitals and may be particularly common on floors and in sink drains. However, C. auris contamination was uncommon in a facility experiencing an outbreak, suggesting that current cleaning and disinfection practices can be effective in minimizing environmental contamination. Additional studies are needed to evaluate the role of contaminated high-touch surfaces in transmission of Candida species other than C. auris. Studies are also needed to investigate the potential for contaminated sink drains and floors to contribute to transmission.
This work was supported by contract 2002011-42039 from the Centers for Disease Control and Prevention (MS) and by the Department of Veterans Affairs.
Curtis J. Donskey has received research grants from Pfizer, GOJO, Clorox, PDI, and Boehringer Laboratories. He is also an associate editor for Pathogens and Immunity. All other authors report no conflicts of interest relevant to this article.
1. Pfaller MA. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin Infect Dis. 1996;22 Suppl 2:S89-94. PubMed PMID: 8722834. doi: 10.1093/clinids/22.supplement_2.s89
2. Arendrup MC, Patterson TF. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis. 2017;216(suppl_3):S445-S51. PubMed PMID: 28911043. doi 10.1093/infdis/jix131
3. Sanchez V, Vazquez JA, Barth-Jones D, Dembry L, Sobel JD, Zervos MJ. Epidemiology of nosocomial acquisition of Candida lusitaniae. J Clin Microbiol. 1992;30(11):3005-8. PubMed PMID: 1360476. Pubmed Central PMCID: 270571.
4. Sanchez V, Vazquez JA, Barth-Jones D, Dembry L, Sobel JD, Zervos MJ. Nosocomial acquisition of Candida parapsilosis: an epidemiologic study. Am J Med. 1993;94(6):577-82. PubMed PMID: 8389525. doi: 10.1016/0002-9343(93)90207-6
5. Vazquez JA, Sanchez V, Dmuchowski C, Dembry LM, Sobel JD, Zervos MJ. Nosocomial acquisition of Candida albicans: an epidemiologic study. J Infect Dis. 1993;168(1):195-201. PubMed PMID: 8515108. doi: 10.1093/infdis/168.1.195
6. Vazquez JA, Dembry LM, Sanchez V, Vazquez MA, Sobel JD, Dmuchowski C, Zervos MJ. Nosocomial Candida glabrata colonization: an epidemiologic study. J Clin Microbiol. 1998;36(2):421-6. PubMed PMID: 9466752. Pubmed Central PMCID: 104553.
7. Lupetti A, Tavanti A, Davini P, Ghelardi E, Corsini V, Merusi I, Boldrini A, Campa M, Senesi S. Horizontal transmission of Candida parapsilosis candidemia in a neonatal intensive care unit. J Clin Microbiol. 2002;40(7):2363-9. PubMed PMID: 12089249. Pubmed Central PMCID: 120610. doi: 10.1128/jcm.40.7.2363-2369.2002
8. Masala L, Luzzati R, Maccacaro L, Antozzi L, Concia E, Fontana R. Nosocomial cluster of Candida guillermondii fungemia in surgical patients. Eur J Clin Microbiol Infect Dis. 2003;22(11):686-8. PubMed PMID: 14566575. doi: 10.1007/s10096-003-1013-4
9. Marol S, Yucesoy M. Molecular epidemiology of Candida species isolated from clinical specimens of intensive care unit patients. Mycoses. 2008;51(1):40-9. PubMed PMID: 18076594. doi: 10.1111/j.1439-0507.2007.01435.x
10. Pinhati HM, Casulari LA, Souza AC, Siqueira RA, Damasceno CM, Colombo AL. Outbreak of candidemia caused by fluconazole resistant Candida parapsilosis strains in an intensive care unit. BMC Infect Dis. 2016;16(1):433. PubMed PMID: 27544427. Pubmed Central PMCID: 4992558. doi: 10.1186/s12879-016-1767-9
11. Dizbay M, Kalkanci A, Sezer BE, Aktas F, Aydogan S, Fidan I, Kustimur S, Sugita T. Molecular investigation of a fungemia outbreak due to Candida parapsilosis in an intensive care unit. Braz J Infect Dis. 2008;12(5):395-9. PubMed PMID: 19219279.
12. Asmundsdottir LR, Erlendsdottir H, Haraldsson G, Guo H, Xu J, Gottfredsson M. Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin Infect Dis. 2008;47(2):e17-24. PubMed PMID: 18549311. doi: 10.1086/589298
13. Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus-United States, May 2013-August 2016. Am J Transplant. 2017;17(1):296-9. PubMed PMID: 28029734. doi: 10.1111/ajt.14121
14. Eyre DW, Sheppard AE, Madder H, Moir I, Moroney R, Quan TP, Griffiths D, George S, Butcher L, Morgan M, Newnham R, Sunderland M, Clarke T, Foster D, Hoffman P, Borman AM, Johnson EM, Moore G, Brown CS, Walker AS, Peto TEA, Crook DW, Jeffery KJM. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N Engl J Med. 2018;379(14):1322-31. PubMed PMID: 30281988. doi: 10.1056/NEJMoa1714373
15. Guerrero DM, Nerandzic MM, Jury LA, Jinno S, Chang S, Donskey CJ. Acquisition of spores on gloved hands after contact with the skin of patients with Clostridium difficile infection and with environmental surfaces in their rooms. Am J Infect Control. 2012;40(6):556-8. PubMed PMID: 21982209. doi: 10.1016/j.ajic.2011.08.002
16. Stiefel U, Cadnum JL, Eckstein BC, Guerrero DM, Tima MA, Donskey CJ. Contamination of hands with methicillin-resistant Staphylococcus aureus after contact with environmental surfaces and after contact with the skin of colonized patients. Infect Control Hosp Epidemiol. 2011;32(2):185-7. PubMed PMID: 21460476. doi: 10.1086/657944
17. Piedrahita CT, Cadnum JL, Jencson AL, Shaikh AA, Ghannoum MA, Donskey CJ. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and Other Candida Species. Infect Control Hosp Epidemiol. 2017;38(9):1107-9. PubMed PMID: 28693657. doi: 10.1017/ice.2017.127
18. Cadnum JL, Shaikh AA, Piedrahita CT, Sankar T, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. Effectiveness of Disinfectants Against Candida auris and Other Candida Species. Infect Control Hosp Epidemiol. 2017;38(10):1240-3. PubMed PMID: 28793937. doi: 10.1017/ice.2017.162
19. Cadnum JL, Shaikh AA, Piedrahita CT, Jencson AL, Larkin EL, Ghannoum MA, Donskey CJ. Relative Resistance of the Emerging Fungal Pathogen Candida auris and Other Candida Species to Killing by Ultraviolet Light. Infect Control Hosp Epidemiol. 2018;39(1):94-6. PubMed PMID: 29157326. doi: 10.1017/ice.2017.239
20. Shams AM, Rose LJ, Edwards JR, Cali S, Harris AD, Jacob JT, LaFae A, Pineles LL, Thom KA, McDonald LC, Arduino MJ, Noble-Wang JA. Assessment of the Overall and Multidrug-Resistant Organism Bioburden on Environmental Surfaces in Healthcare Facilities. Infect Control Hosp Epidemiol. 2016;37(12):1426-32. PubMed PMID: 27619507. Pubmed Central PMCID: 6399740. doi: 10.1017/ice.2016.198
21. Center for Disease Control and Prevention (CDC). (2012, February 08). Stomacher Processing Method: Spore Forming Bacteria [Document. No.: ENV.TE.C.0008 Revision. No.: 1].
22. Jencson AL, Cadnum JL, Piedrahita C, Donskey CJ. Hospital Sinks Are a Potential Nosocomial Source of Candida Infections. Clin Infect Dis. 2017;65(11):1954-5. PubMed PMID: 29020154. doi: 10.1093/cid/cix629
23. Koganti S, Alhmidi H, Tomas ME, Cadnum JL, Jencson A, Donskey CJ. Evaluation of Hospital Floors as a Potential Source of Pathogen Dissemination Using a Nonpathogenic Virus as a Surrogate Marker. Infect Control Hosp Epidemiol. 2016;37(11):1374-7. PubMed PMID: 27523489. doi: 10.1017/ice.2016.181
24. Deshpande A, Cadnum JL, Fertelli D, Sitzlar B, Thota P, Mana TS, Jencson A, Alhmidi H, Koganti S, Donskey CJ. Are hospital floors an underappreciated reservoir for transmission of health care-associated pathogens? Am J Infect Control. 2017;45(3):336-8. PubMed PMID: 28254251. doi: 10.1016/j.ajic.2016.11.005
25. Visnovsky LD, Zhang Y, Leecaster MK, Safdar N, Barko L, Haroldsen C, Mulvey DL, Nevers M, Shaughnessy C, Stratford KM, Drews FA, Samore MH, Mayer J. Effectiveness of a multisite personal protective equipment (PPE)-free zone intervention in acute care. Infect Control Hosp Epidemiol. 2019;40(7):761-6. PubMed PMID: 31172904. doi: 10.1017/ice.2019.111
26. Malhotra P, Khameraj A, Salim T, Armellino D, Wirostek S, Epstein ME, Farber BF. Reengineering the patient's environment: Establishment of a "Red Box" to improve communications with patients on isolation precautions. Am J Infect Control. 2019;47(3):264-7. PubMed PMID: 30413269. doi: 10.1016/j.ajic.2018.09.007
27. Alhmidi H, Cadnum JL, Koganti S, Jencson AL, Rutter JD, Bonomo RA, Wilson BM, Mayer J, Samore MH, Donskey CJ. Shedding of methicillin-resistant Staphylococcus aureus by colonized patients during procedures and patient care activities. Infect Control Hosp Epidemiol. 2019;40(3):328-32. PubMed PMID: 30777587. doi: 10.1017/ice.2018.342
28. Donskey CJ. Beyond high-touch surfaces: Portable equipment and floors as potential sources of transmission of health care-associated pathogens. Am J Infect Control. 2019;47S:A90-A5. PubMed PMID: 31146857. doi: 10.1016/j.ajic.2019.03.017