Brett Williams1, Stefan J. Green2, Ronald J. Bosch3, Ellen S. Chan3, Jeffrey M. Jacobson4, David M. Margolis5, Phillip Engen6, Alan L. Landay7, Cara C. Wilson8, A5286 protocol team
Introduction: HIV-infected individuals have evidence of intestinal microbial translocation which is associated with immune activation and unfavorable clinical outcomes. Rifaximin, a non-absorbable antibiotic which reduces microbial translocation in other disease states, was shown to have a marginal beneficial effect on microbial translocation, T-cell activation, and inflammation in a multisite randomized trial (ACTG A5286; NCT01466595) of HIV-infected persons with poor immunologic recovery receiving ART. Here, we report analysis of the rectal microbiome changes associated with that trial.
Methods: HIV-1-infected individuals receiving ART with CD4-T cell count <350cells/mm3 and viral suppression were randomized 2:1 to rifaximin or no therapy for 4 weeks. Rectal swabs were collected at baseline (pre-treatment) and at week 4 of rifaximin therapy. Genomic DNA extracted from rectal swab samples was analyzed using high throughput sequencing and quantitative PCR of bacterial 16S ribosomal RNA (rRNA) genes.
Results: Forty-eight HIV-infected participants (31 received rifaximin, 17 no treatment) were included. There was broad variability in the recovery of bacterial rRNA from the specimens at baseline. No major significant (FDR P<0.05) effects of rifaximin treatment on alpha- or beta- diversity or individual taxa were observed between or within the treatment arms, with analyses conducted at taxonomic levels from phylum to genus.
Conclusions: Rifaximin did not meaningfully alter the diversity or composition of the rectal microbiome of HIV-infected individuals after 4 weeks of therapy, although rectal swab specimens varied widely in their microbial load.
Keywords: HIV, microbiome, immune activation, microbial translocation, rifaximin
The collection of microorganisms that colonize the gastrointestinal tract of humans, or the gut microbiome, influences many different inflammatory disease processes [1, 2]. In particular, persons living with HIV infection have been reported to have a gut microbiome containing more pro-inflammatory bacteria than seronegative individuals [3-5]. It is hypothesized that this shift in the microbiome, along with other HIV-associated changes in the intestinal mucosa and gut barrier, contributes to the increased microbial translocation and concomitant systemic inflammation and immune activation that characterize all stages of HIV disease [3-5]. Several groups, including the AIDS Clinical Trials Group (ACTG), have demonstrated a link between excess microbial translocation, persistent immune activation, and morbidity and mortality in persons living with HIV [6-8]. Individuals with inadequate peripheral blood CD4+ T-cell recovery (<350cells/mm3) following viral suppression have a particularly notable disrupted gut barrier (zonulin-1, I-FABP) and immune activation as measured by soluble CD14 (sCD14), IL-6 and LPS, which may be due in part to limited intestinal CD4+ T-cell recovery [9-11].
Rifaximin, an oral, non-absorbed antibiotic, reduces circulating lipopolysaccharide (LPS) levels and improves hepatic encephalopathy in persons with cirrhosis [12]. In ACTG A5286, we tested the hypothesis that 4 weeks of rifaximin reduces markers of microbial translocation (including LPS and soluble CD14) and immune activation in HIV-positive individuals with poor CD4 recovery despite full virologic suppression [13]. In that study, rifaximin administration only marginally decreased markers of T-cell activation, microbial translocation, and systemic inflammation. In this secondary analysis, we evaluated whether rifaximin altered the composition or diversity of gut resident microbial communities in a subset of A5286 participants with available paired rectal swabs.
ACTG A5286 was a 4-week, randomized, open-label study in which 65 eligible individuals who were HIV positive, with CD4 <350c/mm3, with plasma HIV RNA below the limit of detection, and receiving ART for at least 48 consecutive weeks were randomized to receive rifaximin 550 mg by mouth 2 times daily for 4 weeks or no treatment. The full study inclusion and exclusion criteria and the primary results have been previously reported [13]. Institutional review board approval was obtained by each ACTG site. Participants provided written informed consent.
The effect of rifaximin on the gut microbiome was evaluated in available pre-treatment (baseline) and post-treatment (week 4) anal swab samples selected from the primary analysis population of ACTG A5286.
Rectal swabs were inserted into the anal canal, beyond the anal verge (±3 cm), with five circular motions in performing the collection. Then, the rectal swabs were snap frozen in liquid nitrogen and stored at -80°C until use.
Genomic DNA was extracted from rectal swab samples using the FastDNA Spin Kit for Soil, (MP Biomedicals, Solon, OH), according to the manufacturer’s instructions. Quantification of bacterial small subunit rRNA (SSU or 16S rRNA) gene abundance was performed as described previously using Taqman 2× Gene Expression Master Mix (Invitrogen, Foster City, CA) [14]. Primers and probe were ordered from Integrated DNA Technologies (Coralville, IA). Absolute quantification was performed using a standard curve derived from PCR products generated by near-full gene amplification of 16S rRNA genes using the general bacterial primer set 27F and 1492R.The standard curve was linear across a scale of 7 orders of magnitude (from 6.85E+01 to 6.85E+08 copies/reaction), with an efficiency of 84%. Assays were performed in triplicate in 384-well plates in a volume of 10 uL per sample, using a ViiA7 real-time PCR instrument (Life Technologies). All qPCR assays were performed at the University of Illinois at Chicago Sequencing Core (UICSQC).
Fecal microbiomes were characterized using deep sequencing of PCR-amplified portions of microbial 16S rRNA genes. Sequencing was performed at Argonne National Laboratory, using the standard Earth Microbiome Project primers (ie, 515F/806R) containing sample-specific barcode sequences and Illumina adapter sequences [15]. Amplicons were pooled and sequenced on an Illumina MiSeq sequencer, implementing V2 chemistry with paired-end 2x150 base reads.
Forward and reverse reads were merged and were quality trimmed, and sequences longer than 200 bases were exported (v7.0, CLC Bio, Qiagen, Boston, MA). Sequences were screened for chimeras (usearch61 algorithm), and putative chimeric sequences were removed from the dataset (QIIME v1.8.0) [16,17]. Each sample sequence set was sub-sampled (rarefied) to 6,500 sequences and data were pooled, renamed, and clustered into operational taxonomic units (OTU) at 99% similarity (usearch61algorithm) [18]. A depth of 6,500 sequences was used as this number represented approximately 75% of the sequences from the sample with the fewest sequences. Representative sequences from each OTU were extracted and classified using the uclust consensus taxonomy assigner (Greengenes 13_8 reference database). A biological observation matrix (BIOM; a taxon-by-sample abundance table) was generated at each taxonomic level (“make OTU table” algorithm) and analyzed and visualized using the software package Primer 6 [19-21]. To compare between samples, the Bray-Curtis metric was used, as implemented within Primer 6. Comparisons between different treatment groups and sampling visits were performed using analysis of similarity (ANOSIM) at various taxonomic levels. Data were standardized within samples and then square-root transformed. Differences in the relative abundance of individual taxa between a priori defined treatment or visit groups were detected using a Kruskal-Wallis test generating a Benjamini-Hochberg false-discovery rate (FDR) corrected P-value. The FDR adjustment is used to address type I errors (false positives) when dealing with a large number of variables in a single analysis. For microbial community analyses, analyses are performed to detect significant differences in the relative abundance of many taxa, and in these studies, approaches such as the Bonferroni correction have been too stringent [22]. Taxa with an average abundance of <1% across the sample set were removed from these analyses.
Alpha diversity indices, which represent within-sample diversity measures (eg, number of taxa present, evenness of relative abundance of taxa, and hybrid measures of both–such as the Shannon Index) were calculated using the software package Primer 6. Analyses were performed at the taxonomic level of genus, as the short-read amplicon sequence data (ie, 252-253 bases in length for each sequence) are not sufficiently long for consistent annotation at the species level. Most sequences could be annotated at the taxonomic level of genus, and this taxonomic level was therefore used for comparative analyses of diversity. Differences in the alpha diversity indices between groups were tested for significance using the Mann-Whitney non-parametric U-test implemented within the software package Origin (Origin2015). Furthermore, comparison of between-visit microbial community structure was performed using the Bray-Curtis metric, and the distribution of baseline vs week 4 similarity within participants from the Rifaximin and the no-treatment arms were measured. A Mann-Whitney test was performed to determine if the shift in community from baseline to week 4 was significantly different between study arms. Stacked histograms were generated for visualization purposes in Origin.
A series of ANOSIM calculations were performed at multiple taxonomic levels to determine if microbial community structure was significantly different between study arms, and between visits within study arms. In addition, group-significance (Kruskal-Wallis) test results, run at all taxonomic levels from phylum to species, were compiled. Significance was defined as FDR P<0.05.
Analysis was an as-treated analysis that was limited to participants in both arms who had data for baseline and week 4, and (for the rifaximin arm) who remained on study treatment through week 4 (allowing ≤ 6 missed doses). These participants did not change ART, use prohibited medications, or have virologic failure during this time period. All statistical tests were 2-sided at the 0.05 nominal level of significance without adjustments for multiple testing.
Participants in A5286 who provided rectal swab samples from both visits and from whose samples at least 6,500 sequences were generated, were included in the analysis. After removing participants whose samples did not meet these criteria, 48 participants (31 from the rifaximin arm; 17 from the no-treatment arm) had complete data for this analysis (Table 1), yielding a total of 96 samples for analysis. The rifaximin and no-treatment groups were similar in baseline characteristics. Overall, the median age was 50 (years), 90% male, 52% white non-Hispanic, 31% black non-Hispanic, and 15% Hispanic. The median CD4 count was 229 and duration of undetectable viral load was 3.3 years.
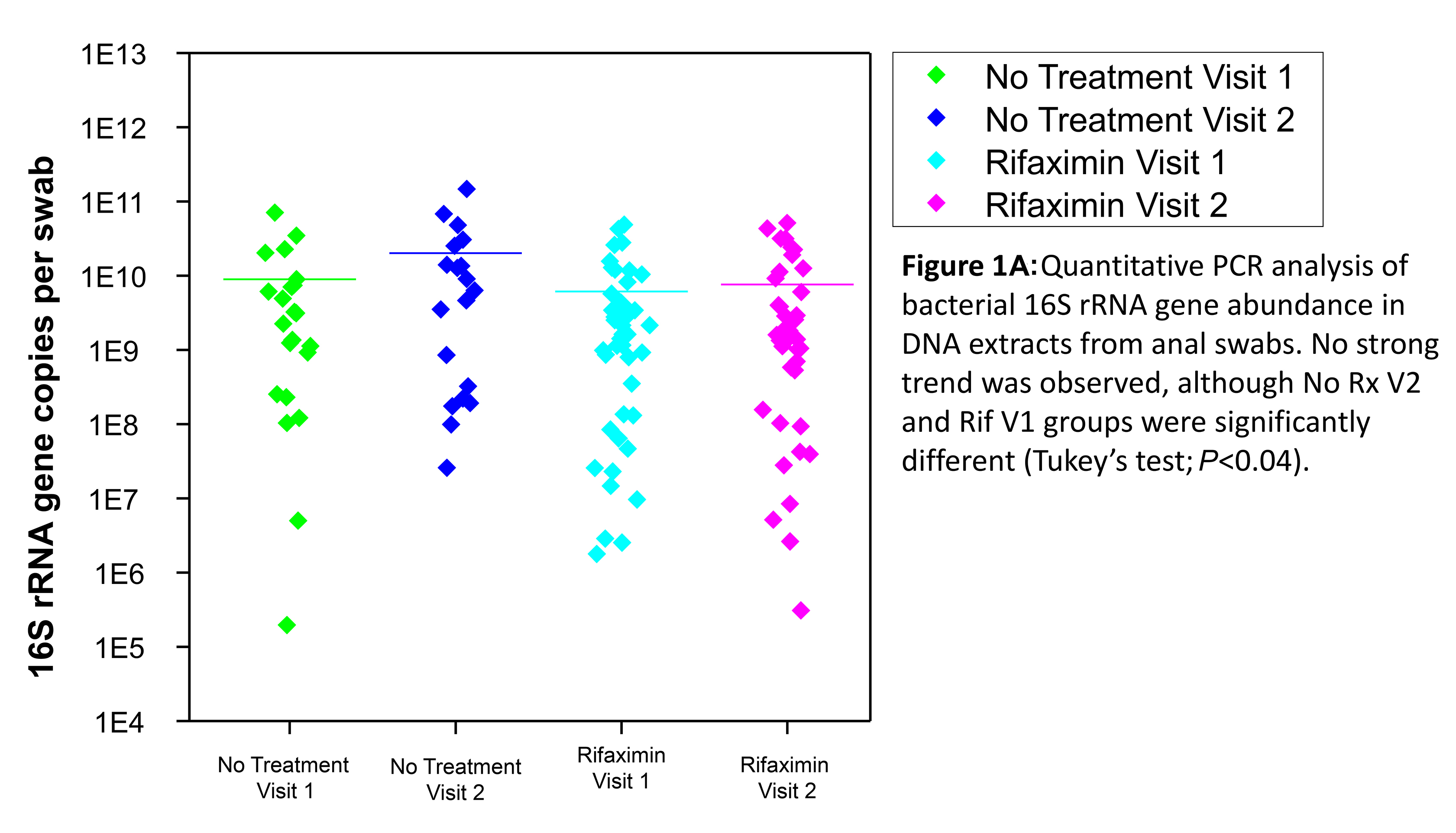
Quantitative analysis of bacterial 16S rRNA gene abundance was performed using quantitative PCR to determine if rifaximin treatment resulted in a shift in the abundance of bacteria in each sample. Total bacterial 16S rRNA gene abundance was not significantly different following treatment with rifaximin, although the absolute abundance in samples varied across 7 orders of magnitude (Figure 1A). The variability between time points was similar in the rifaximin and no- treatment groups; it is possible that small variations due to rifaximin may have been obscured by the relatively high variation associated with rectal swabs as a collection method.

Figure 1A . Quantitative PCR analysis of bacterial 16S rRNA gene abundance in DNA extracts from anal swabs. No strong trend was observed, although no-treatment visit 2 and Rifaximin visit 1 groups were significantly different (Tukey’s test; P<0.04).
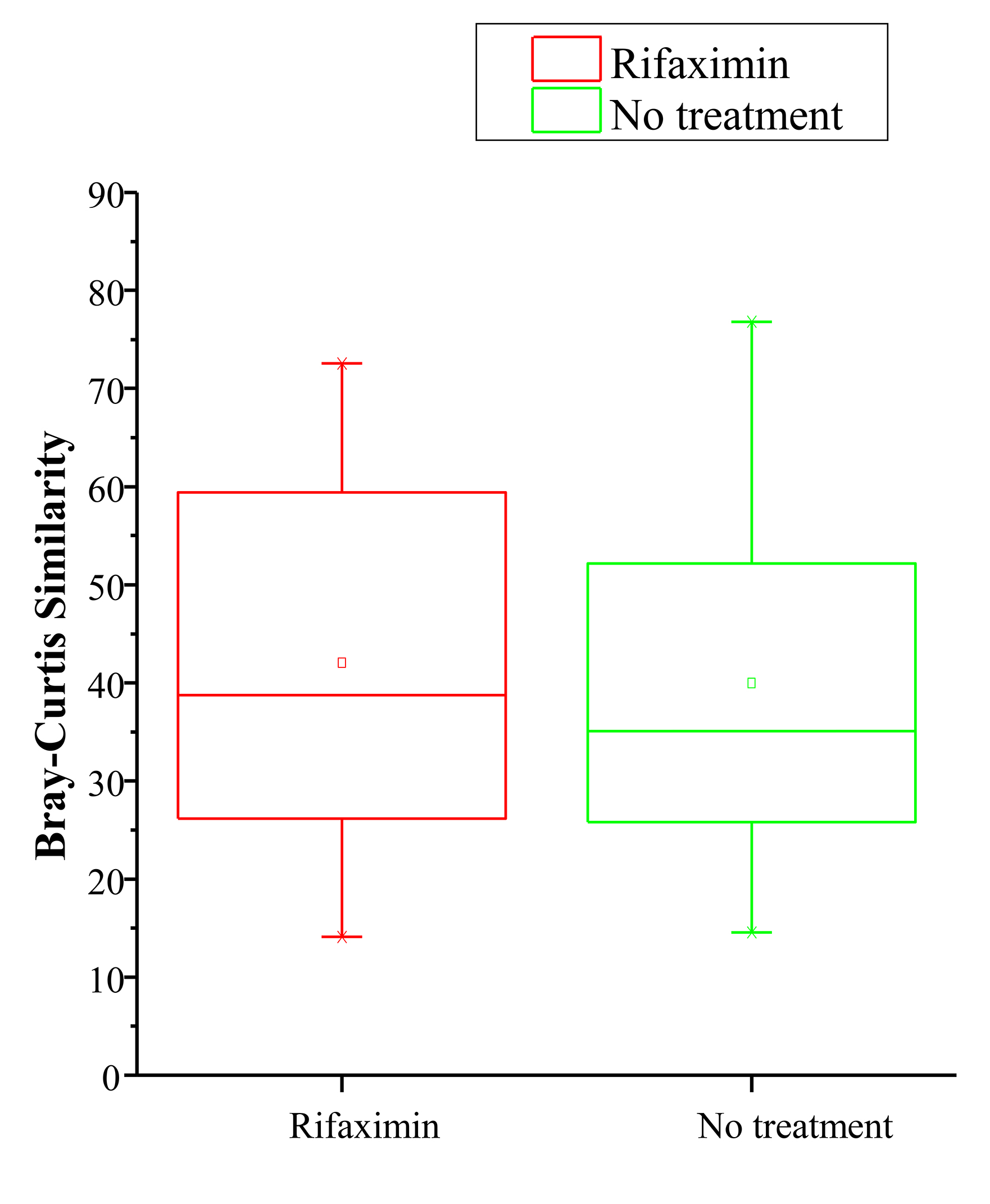
To determine if microbial community composition between visits within each individual was more variable in the no-treatment or rifaximin arms, Bray-Curtis similarity values for each individual (baseline vs week 4) were calculated. No significant difference was observed for the within-group comparison of these values, suggesting that rifaximin did not alter the variability of the microbial community composition between visits.
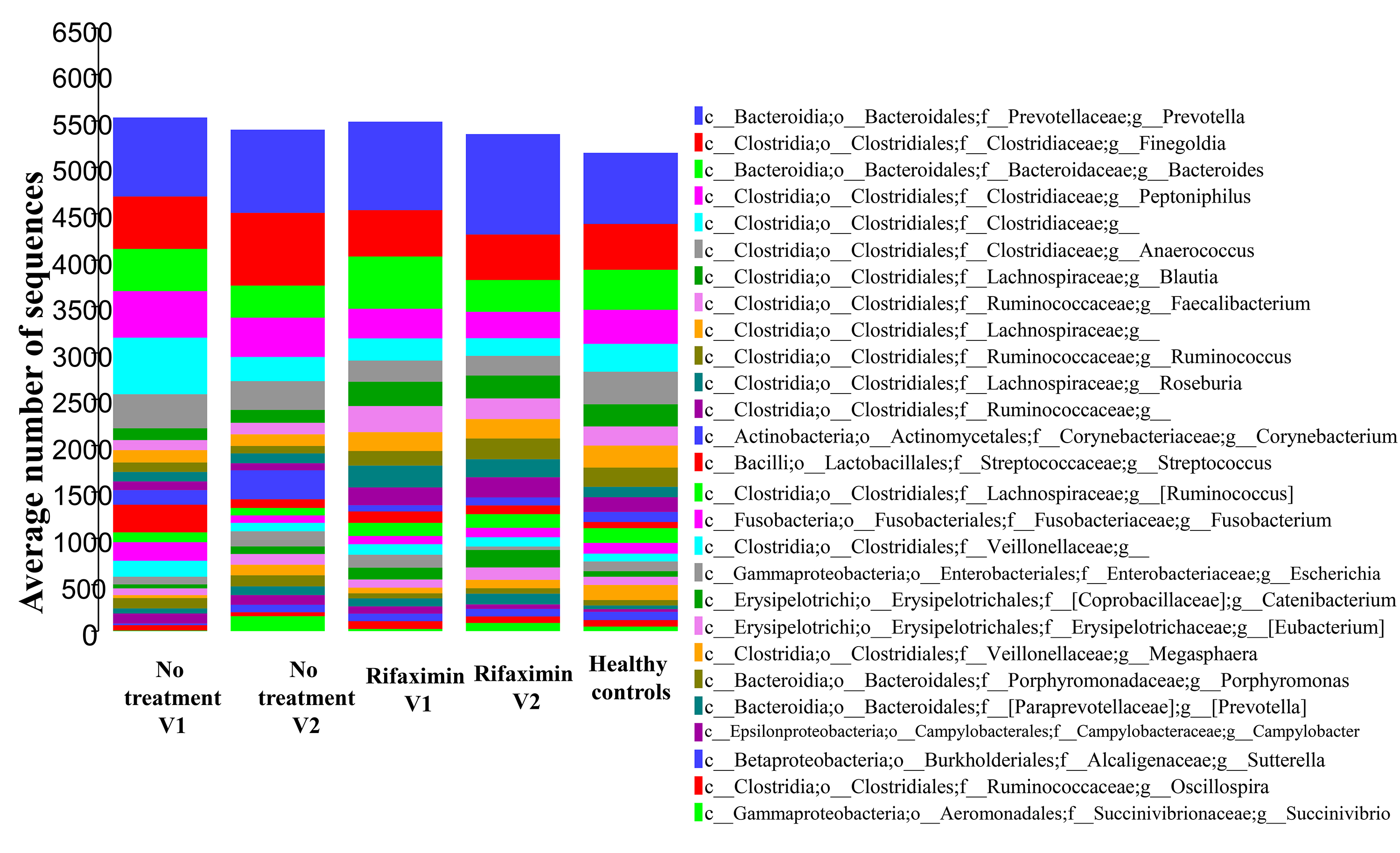
Similarly, we assessed microbial community diversity to determine if there were significant changes in alpha diversity indices between treatment arms and between visits within treatment arms. An analysis of the Shannon index (a combined measure of taxon richness and evenness) between groups did not reveal a systematic shift in diversity between treatments or visits (Figure 1B). A significant difference in Shannon index (taxonomic level of genus) was observed between control and rifaximin arm samples at visit 2 (4 weeks), which appears to have been driven by a trend toward increased alpha diversity in the rifaximin arm at baseline. No difference was seen at the species level. In each of the groups, sequences from bacteria of the phylum Firmicutes predominated (group average relative abundance ranged from 53.3%-62.5%), followed by Bacteroidetes (22.3%-27.6%) and Proteobacteria (5.6%-10.5%) (Figure 1C). The genus Prevotella was the most prevalent genus (11.8%-16.7%) in all groups at all time points.
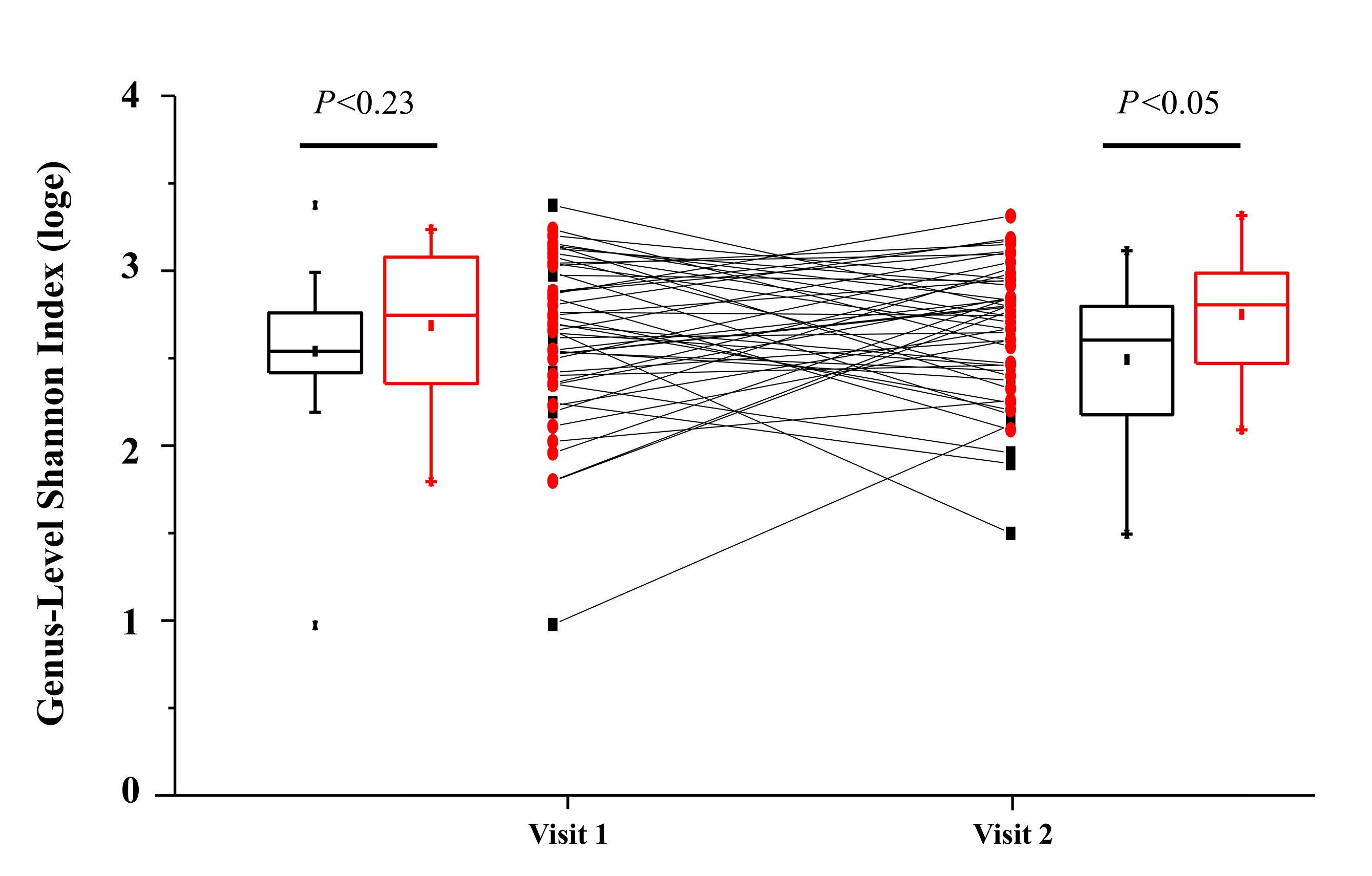
Figure 1B. Change in fecal microbial diversity between visit 1 (before treatment) and visit 2 (after treatment) for individuals taking rifaximin (red circles) and for individuals taking no study treatment (black squares). No significant difference in Shannon Index between treatment groups was observed at baseline (visit 1; Mann-Whitney U-test), but a significant difference was observed at visit 2.
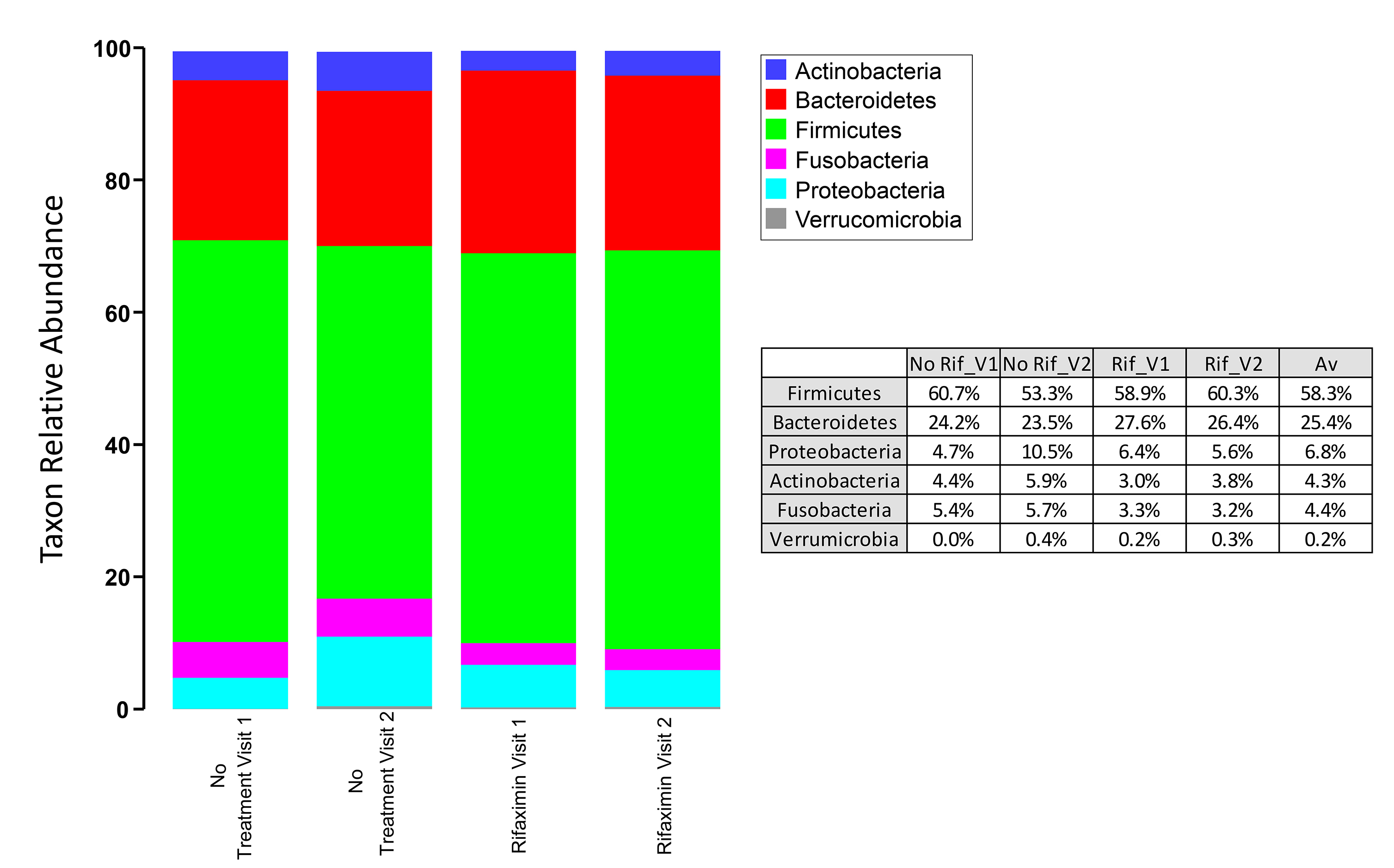
Figure 1C. Phylum-level microbial community composition of fecal microbiome samples from individuals in the rifaximin and placebo experimental arms. Average abundance of each taxon is shown among 31 individuals (rifaximin arm) and 17 individuals (no-treatment arm). Each sample was rarefied to 6500 sequences and average values for each taxon are shown. No taxa are significantly different between control and rifaximin arms at visit 2. Greater than 99% of all sequences belonged to 1 of the 6 phyla shown.
No significant difference in microbial community structure within treatment arms between baseline and week 4 was observed (ANOSIM global R values -0.022 to -0.029; P-values 0.811-0.925). At the second visit, the microbial communities of the no-treatment control and rifaximin arms were significantly different, (ANOSIM global R=0.099; P-value = 0.041) and this was similar to what was seen with the Shannon diversity index. At both baseline and week 4, there were no differences in the phylum or species level assessment, and only 1 genus level difference was identified between the 2 groups: the genus Sutterella was more abundant in the rifaximin arm at baseline (FDR value <0.05). The average relative abundance of sequences for each group annotated as Sutterella ranged from 0.3% to 1.4%. However, no individual taxa were significantly different between the 2 treatment arms at week 4 (FDR P-value <0.05).
After 4 weeks of rifaximin therapy, gut microbiome diversity and composition did not change significantly in a cohort of HIV-infected persons with suppressed HIV viremia and CD4 counts below 350 cells/mm3. At week 4, there was a modest difference in genus level diversity as measured by the Shannon index between the rifaximin and the no-treatment groups although this difference likely reflects differences present at baseline. The difference in the genus Sutterella at baseline which disappeared following treatment is unlikely to be meaningful as it was not associated with any other major changes in the microbiome over time. Lack of substantial change in rectal microbiota between baseline and week 4 in the rifaximin group may explain the marginal effect of rifaximin therapy on markers of microbial translocation and immune activation seen in the parent study [13]. The parent study was designed based on findings that endotoxemia was driving persistent immune activation and that rifaximin therapy resulted in reductions in endotoxemia in participants with hepatic encephalopathy, however these findings were based on 8 weeks of rifaximin therapy [23]. In the hepatic encephalopathy study mentioned above, no major changes were noted in the microbial abundance after 8 weeks of therapy, but alterations in gut bacterial linkages to metabolites were noted [23]. Previous studies also showed that antibiotic therapy reduced serum LPS levels, a marker of microbial translocation, in SIV-infected nonhuman primates [6]. More recently, combination therapy with rifaximin and sulfasalazine was demonstrated to reduce microbial translocation and immune activation as well as prevent mucosal CD4 depletion in acute SIV infection [24]. Conversely, no significant effect on microbial translocation or immune activation was noted when this combination was given to chronically SIV-infected animals, consistent with findings from the A5286 parent study which included chronically infected humans [13, 24]. Thus, an alternative explanation is that in chronic infection, and particularly in persons with advanced disease (ie, low nadir CD4 count), rifaximin is not an effective intervention as the changes to gut mucosal integrity and immune surveillance are beyond repair.
While Pandrea et al did not measure alterations in the fecal microbiota in their main study, they did demonstrate, using a sub-population, that 2 weeks of rifaximin treatment of SIV-naive animals resulted in changes in the fecal microbiota composition of stool, a significant reduction in fecal biodiversity, and reduced fecal bacterial loads [24]. Specifically, changes in fecal microbiome composition were dominated by an increase in the abundance of Prevotellaceae [24]. The inability of rifaximin to reduce inflammation during chronic infection may be related to severe pre-existing mucosal immune disruption and a resultant dysbiosis, associated with an increase in Prevotellaceae, which may only be detectable in stool or more proximal samples [3,4,25, 26]. However, one large cohort study suggested that the increase in Prevotellaceae may be an enterotype found in men who have sex with men (MSM) rather than being related to HIV infection [27.
This study had several limitations. First, our sample size was relatively small, although on par with several prior HIV specific microbiome studies [3-5, 26, 28, 29, 30]. The intervention was of relatively short duration and any change that occurred after the week 4 visit would have been missed. The microbiome analysis was conducted at 4 weeks while the primary published analysis, where the authors showed that the use of rifaximin led to a reduction in endotoxemia, was based on an 8-week analysis. It would have been more appropriate to analyze the changes in the microbiome at the same time point, but samples are not available from the 8-week time point.
Data on potentially confounding factors such as diet, anal sex, and tobacco or alcohol intake were not collected, though the effect of these factors should have been mitigated by randomization. Two of our findings suggest that the particular method of obtaining rectal swabs used for this study may result in too much variability. Bray-Curtis similarity comparing baseline to the week 4 microbiome (Figure 1B) was quite low (<40%) in the no-treatment group, suggesting poor within-subject reproducibility over time compared to prior publications, and the bacterial load per swab varied by approximately 4 logs between samples. These findings suggest that swab-to-swab variability was high and may be dependent on factors such as time between last bowel movement and swab collection technique. Because this was a multi-site study, all rectal swabs were not collected by the same individual and there was likely variability in technique. The high swab-to-swab variability likely reduced our power to measure any small changes caused by rifaximin. Single-center studies have found rectal swab microbiota sampling to be fairly reliable and to correlate well with more proximal gut samples [31, 32]. Future studies should evaluate the validity and reproducibility of rectal swab specimens for intestinal microbiome analysis using a comparison between rectal swab, stool, and sigmoid biopsy samples in both healthy participants and those with pathology.
We failed to identify any effects of 4 weeks of rifaximin on the rectal microbiome. Our results appear to confirm those of groups investigating rifaximin in human cohorts with hepatic encephalopathy, irritable bowel syndrome, or inflammatory bowel disease (IBD) which found minimal or no change in the microbiome following rifaximin therapy [21, 32, 34]. The change in microbiota induced by rifaximin may be temporary as at least some microbes can quickly develop resistance to rifaximin; thus, we may have missed this change by sampling only after 4 weeks of treatment [35]. For example, Pandrea et al noted a change at 2 weeks in their SIV model [24]. Sub-inhibitory levels of rifaximin have been demonstrated to downregulate virulence factors and the IL-8 induction potential of gastrointestinal pathogenic Enterobacteriaceae [36]. This may suggest that gut bacteria may rapidly adapt to survive the presence of rifaximin, yet continue to have altered expression of virulence factors, which could explain the therapeutic effects seen in hepatic encephalopathy and IBD. The symptomatic improvement seen in traveler's diarrhea with rifaximin despite failure to eradicate the causative strain of Enterobacteriaceae also suggests that rifaximin may be better at downregulating virulence factors than it is at killing [37,38].
Rifaximin did not significantly alter the composition or diversity of the colon microbiome of HIV-infected individuals as assessed by rectal swabs, although specimens varied widely in their microbial load and composition. Future studies of the colon microbiome should compare rectal swab samples to more proximal samples. Further investigation of microbial metabolites and virulence factors may be helpful in elucidating the mechanisms by which rifaximin reduces microbial translocation in other disease states.
We thank the A5286 participants, the ACTG clinical trial sites that enrolled and followed up the participants, and the ACTG. The other members of the A5286 team, besides the authors, include John W. Mellors, MD (virologist); Ali Keshavarzian, MD, Benigno Rodriguez, MD, Mariam Aziz, MD, Rachel Presti, MD, Steven Deeks, MD (investigators); Ruth Ebiasah, PharmD, MS, RPh (DAIDS pharmacist); Laurie Myers, MS, MT (data manager); LuAnn Borowski, MSc, Jill Plants, BS (laboratory technologists); David A. Palm, MS (CSS representative); Derek Weibel, MS Ed (laboratory data manager); and Beverly Putnam, RN, ANP (field representative). The following sites participated in A5286: Alabama Therapeutics Clinical Research Site (CRS) (Elizabeth Lindsey, RN, and Amy Player, PharmD), Beth Israel Deaconess Medical Center ACTG CRS (Mary Albrecht, MD, and Andrea Kershaw, NP), Brigham and Women's Hospital ACTG CRS (Paul Sax, MD, and Cheryl Keenan, RN), Case CRS (Patricia Walton, RN, and Jane Baum, RN), Cornell CRS (Todd Stroberg, RN, and Valery Hughes, NP), Duke University Medical Center Adult CRS, Georgetown University CRS (Laura Coster, MD, and Princy N. Kumar, MD), HIV Prevention & Treatment CRS (Michael T. Yin and Jolene Noel-Connor), Hospital of the University of Pennsylvania CRS (Pablo Tebas, MD, and Aleshia Thomas, RN, BSN), Institute of Human Virology Baltimore Treatment CRS (Charles E. Davis, Jr, MD, and Robert R. Redfield, Jr, MD), Massachusetts General Hospital ACTG CRS (Amy Sbrolla, RN, and Teri Flynn, ANP), MetroHealth CRS (Traci Davis, RN, and Kim Whitely, RN), New Jersey Medical School CRS (Baljinder Singh, MA, and Shobha Swaminathan, MD), Northwestern University CRS (Donna McGregor and Frank Palella, MD), NYU and Bellevue HIV/AIDS Clinical Trials Unit (Judith Aberg, MD, and Karen Cavanagh, RN), Puerto Rico–ACTU (Jorge L. Santana Bagur, MD, and Olga Méndez Flores, MD), Rush CRS (Janice Fritsche and Beverly Sha, MD), Stanford CRS (Debbie Slamowitz, RN, ACRN, and Sandra Valle, PA-C), The Miriam Hospital ACTG CRS (Karen Tashima, MD, and Helen Patterson, LPN), Ohio State University AIDS CRS (Heather Harber, RN, and Michael Para, MD), The Ponce de Leon Center CRS (Molly Eaton, MD, and Dale Maddox, RN, BSN), UCLA CARE Center CRS (Judith Currier, MD, and Vanessa Cajahuaringa), UCSF AIDS CRS (Annie Luetkemeyer, MD, and Jay Dwyer, RN), University of Cincinnati CRS (Carl J. Fichtenbaum, MD, and Michelle Saemann, RN), University of Colorado Hospital CRS (Graham Ray and Thomas Campbell), University of Miami HIV/ACTU (Margaret A. Fischl, MD, and Hector Bolivar, MD), University of North Carolina AIDS CRS (Jonathan Oakes and Miriam Chicurel-Bayard), University of Pittsburgh CRS (Christine Tripoli, BSN, and D. Renee Weinman, BS), University of Rochester/Trillium Health (Mary Adams, RN, and Christine Hurley, RN), University of Washington AIDS CRS (Shelia Dunaway, MD, and Sheryl Storey, PA-C), and Washington University CRS (Michael Klebert, PhD, RN, ANP-BC, and Michael Royal, BS, RPh).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This work was supported by grants from the National Institutes of Health (AI068636 to the ACTG Network Leadership; AI068634 and AI068636 to ACTG Statistical Data Analysis Center [E. S. C. and R. J. B.]; AI068636 to A. L. L.; AI069452, A1025439, AI069424, AI069447, AI069419, AI069418, AI069532, AI069470, AI069494, AI069511-08, AI069439, AI069471, AI069418, AI069556, AI069477, AI069494, AI069439, AI069501, AI069471, AI069412, AI069412, AI069412, AI069502, AI069494, AI69501, AI069423, AI069415, AI069481, AI069534, AI069503 and AI069432 to the ACTG clinical research sites; TR001082, RR024160, TR001070, TR000439 and TR001111 to the Clinical and Translational Science Centers; and Al050404, AI073961, AI50410 and AI045008 to the Centers for AIDS Research).
Jeffrey M. Jacobson, David M. Margolis, and Alan L. Landay are associate editors for Pathogens and Immunity.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
1. Papa E, Docktor M, Smillie C, Weber S, Preheim SP, Gevers D, Giannoukos G, Ciulla D, Tabbaa D, Ingram J, Schauer DB, Ward DV, Korzenik JR, Xavier RJ, Bousvaros A, Alm EJ. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One. 2012;7(6):e39242. PubMed PMID: 22768065. Pubmed Central PMCID: 3387146. doi: 10.1371/journal.pone.0039242
2. Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda). 2016;31(4):283-93. PubMed PMID: 27252163. doi: 10.1152/physiol.00041.2015
3. Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7(4):983-94. PubMed PMID: 24399150. Pubmed Central PMCID: 4062575. doi: 10.1038/mi.2013.116
4. Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, Cox S, Engen P, Chakradeo P, Abbasi R, Gorenz A, Burns C, Landay A. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829. PubMed PMID: 24586144. Pubmed Central PMCID: 3930561. doi: 10.1371/journal.ppat.1003829
5. Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211(1):19-27. PubMed PMID: 25057045. Pubmed Central PMCID: 4326316. doi: 10.1093/infdis/jiu409
6. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365-71. PubMed PMID: 17115046. doi: 10.1038/nm1511
7. Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248-59. PubMed PMID: 24795473. Pubmed Central PMCID: 4192039. doi: 10.1093/infdis/jiu254
8. Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228-38. PubMed PMID: 24755434. Pubmed Central PMCID: 4192038. doi: 10.1093/infdis/jiu238
9. Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, Gripshover B, Salata RA, Taege A, Lisgaris M, McComsey GA, Kirchner E, Baum J, Shive C, Asaad R, Kalayjian RC, Sieg SF, Rodriguez B. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204(8):1217-26. PubMed PMID: 21917895. Pubmed Central PMCID: 3218674. doi: 10.1093/infdis/jir507
10. Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, Morace G, Gori A, Monforte AD. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22(15):2035-8. PubMed PMID: 18784466. doi: 10.1097/QAD.0b013e3283112d29
11. Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77(21):11708-17. PubMed PMID: 14557656. Pubmed Central PMCID: 229357. doi: 10.1128/jvi.77.21.11708-11717.2003
12. Vlachogiannakos J, Saveriadis AS, Viazis N, Theodoropoulos I, Foudoulis K, Manolakopoulos S, Raptis S, Karamanolis DG. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009;29(9):992-9. PubMed PMID: 19210289. doi: 10.1111/j.1365-2036.2009.03958.x
13. Tenorio AR, Chan ES, Bosch RJ, Macatangay BJ, Read SW, Yesmin S, Taiwo B, Margolis DM, Jacobson JM, Landay AL, Wilson CC, Team A. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis. 2015;211(5):780-90. PubMed PMID: 25214516. Pubmed Central PMCID: 4334803. doi: 10.1093/infdis/jiu515
14. Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148(Pt 1):257-66. PubMed PMID: 11782518. doi: 10.1099/00221287-148-1-257
15. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621-4. PubMed PMID: 22402401. Pubmed Central PMCID: 3400413. doi: 10.1038/ismej.2012.8
16. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460-1. PubMed PMID: 20709691. doi: 10.1093/bioinformatics/btq461
17. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335-6. PubMed PMID: 20383131. Pubmed Central PMCID: 3156573. doi: 10.1038/nmeth.f.303
18. Gihring TM, Green SJ, Schadt CW. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ Microbiol. 2012;14(2):285-90. PubMed PMID: 21923700. doi: 10.1111/j.1462-2920.2011.02550.x
19. McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, Wilke A, Huse S, Hufnagle J, Meyer F, Knight R, Caporaso JG. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience. 2012;1(1):7. PubMed PMID: 23587224. Pubmed Central PMCID: 3626512. doi: 10.1186/2047-217X-1-7
20. Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, Turek FW, Keshavarzian A. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9(5):e97500. PubMed PMID: 24848969. Pubmed Central PMCID: 4029760. doi: 10.1371/journal.pone.0097500
21. Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 1993;18:117-143. doi: 10.1111/j.1442-9993.1993.tb00438.x
22. Benjamini, Y. and Hochberg, Y., 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), pp.289-300.
23. Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042. PubMed PMID: 23565181. Pubmed Central PMCID: 3615021. doi: 10.1371/journal.pone.0060042
24. Pandrea I, Xu C, Stock JL, Frank DN, Ma D, Policicchio BB, He T, Kristoff J, Cornell E, Haret-Richter GS, Trichel A, Ribeiro RM, Tracy R, Wilson C, Landay AL, Apetrei C. Antibiotic and Antiinflammatory Therapy Transiently Reduces Inflammation and Hypercoagulation in Acutely SIV-Infected Pigtailed Macaques. PLoS Pathog. 2016;12(1):e1005384. PubMed PMID: 26764484. Pubmed Central PMCID: 4713071. doi: 10.1371/journal.ppat.1005384
25. Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14(3):329-39. PubMed PMID: 24034618. Pubmed Central PMCID: 3864811. doi: 10.1016/j.chom.2013.08.006
26. Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, Deeks SG, Hunt PW, Lynch SV, McCune JM. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5(193):193ra91. PubMed PMID: 23843452. Pubmed Central PMCID: 4094294. doi: 10.1126/scitranslmed.3006438
27. Noguera-Julian M, Rocafort M, Guillen Y, Rivera J, Casadella M, Nowak P, Hildebrand F, Zeller G, Parera M, Bellido R, Rodriguez C, Carrillo J, Mothe B, Coll J, Bravo I, Estany C, Herrero C, Saz J, Sirera G, Torrela A, Navarro J, Crespo M, Brander C, Negredo E, Blanco J, Guarner F, Calle ML, Bork P, Sonnerborg A, Clotet B, Paredes R. Gut Microbiota Linked to Sexual Preference and HIV Infection. EBioMedicine. 2016;5:135-46. PubMed PMID: 27077120. Pubmed Central PMCID: 4816837. doi: 10.1016/j.ebiom.2016.01.032
28. Yang L, Poles MA, Fisch GS, Ma Y, Nossa C, Phelan JA, Pei Z. HIV-induced immunosuppression is associated with colonization of the proximal gut by environmental bacteria. AIDS. 2016;30(1):19-29. PubMed PMID: 26731752. Pubmed Central PMCID: 4813506. doi: 10.1097/QAD.0000000000000935
29. McHardy IH, Li X, Tong M, Ruegger P, Jacobs J, Borneman J, Anton P, Braun J. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1(1):26. PubMed PMID: 24451087. Pubmed Central PMCID: 3971626. doi: 10.1186/2049-2618-1-26
30. Perez-Santiago J, Gianella S, Massanella M, Spina CA, Karris MY, Var SR, Patel D, Jordan PS, Young JA, Little SJ, Richman DD, Smith DM. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27(12):1921-31. PubMed PMID: 24180001. Pubmed Central PMCID: 3816380. doi: 10.1097/qad.0b013e3283611816
31. Budding AE, Grasman ME, Eck A, Bogaards JA, Vandenbroucke-Grauls CM, van Bodegraven AA, Savelkoul PH. Rectal swabs for analysis of the intestinal microbiota. PLoS One. 2014;9(7):e101344. PubMed PMID: 25020051. Pubmed Central PMCID: 4096398. doi: 10.1371/journal.pone.0101344
32. Bassis CM, Moore NM, Lolans K, Seekatz AM, Weinstein RA, Young VB, Hayden MK, Program CDCPE. Comparison of stool versus rectal swab samples and storage conditions on bacterial community profiles. BMC Microbiol. 2017;17(1):78. PubMed PMID: 28359329. Pubmed Central PMCID: 5374586. doi: 10.1186/s12866-017-0983-9
33. Maccaferri S, Vitali B, Klinder A, Kolida S, Ndagijimana M, Laghi L, Calanni F, Brigidi P, Gibson GR, Costabile A. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother. 2010;65(12):2556-65. PubMed PMID: 20852272. doi: 10.1093/jac/dkq345
34. Soldi S, Vasileiadis S, Uggeri F, Campanale M, Morelli L, Fogli MV, Calanni F, Grimaldi M, Gasbarrini A. Modulation of the gut microbiota composition by rifaximin in non-constipated irritable bowel syndrome patients: a molecular approach. Clin Exp Gastroenterol. 2015;8:309-25. PubMed PMID: 26673000. Pubmed Central PMCID: 4675645. doi: 10.2147/CEG.S89999
35. Kothary V, Scherl EJ, Bosworth B, Jiang ZD, Dupont HL, Harel J, Simpson KW, Dogan B. Rifaximin resistance in Escherichia coli associated with inflammatory bowel disease correlates with prior rifaximin use, mutations in rpoB, and activity of Phe-Arg-beta-naphthylamide-inhibitable efflux pumps. Antimicrob Agents Chemother. 2013;57(2):811-7. PubMed PMID: 23183443. Pubmed Central PMCID: 3553721. doi: 10.1128/AAC.02163-12
36. Jiang ZD, Ke S, Dupont HL. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents. 2010;35(3):278-81. PubMed PMID: 20045287. doi: 10.1016/j.ijantimicag.2009.11.013
37. Taylor DN, Bourgeois AL, Ericsson CD, Steffen R, Jiang ZD, Halpern J, Haake R, Dupont HL. A randomized, double-blind, multicenter study of rifaximin compared with placebo and with ciprofloxacin in the treatment of travelers' diarrhea. Am J Trop Med Hyg. 2006;74(6):1060-6. PubMed PMID: 16760520.
38. DuPont HL, Jiang ZD, Ericsson CD, Adachi JA, Mathewson JJ, DuPont MW, Palazzini E, Riopel LM, Ashley D, Martinez-Sandoval F. Rifaximin versus ciprofloxacin for the treatment of traveler's diarrhea: a randomized, double-blind clinical trial. Clin Infect Dis. 2001;33(11):1807-15. PubMed PMID: 11692292. doi: 10.1086/323814