Netanya S. Utay1, Anoma Somasunderam1, John E. Hinkle2, Bryon W. Petschow3, Christopher J. Detzel3, Ma Somsouk4, Carl J. Fichtenbaum5, Eric M. Weaver3, Audrey L. Shaw3, David M. Asmuth6
1
Department of Medicine, McGovern Medical School, University of Texas Health
Science Center at Houston, Houston, Texas
2
EarlyPhase Sciences, Inc., Magnolia, Texas
3
Entera Health, Inc., currently located at 2425 Oak Tree Ct., Ankeny, Iowa
4
Department of Medicine, University of California, San Francisco, San
Francisco, California
5
Department of Medicine, University of Cincinnati, Cincinnati, Ohio
6
Department of Medicine, University of California Davis Medical Center,
Sacramento, California
Netanya S. Utay
713-500-6714
netanya.s.utay@uth.tmc.edu
Background: Systemic inflammation persists in chronic HIV infection and is associated with increased rates of non-AIDS events such as cardiovascular and liver disease. Increased gut permeability and systemic exposure to microbial products are key drivers of this inflammation. Serum-derived bovine immunoglobulin/protein isolate (SBI) supports gut healing in other conditions such as inflammatory bowel disease.
Methods: In this randomized, double-blind study, participants receiving suppressive antiretroviral therapy (ART) with chronic diarrhea received placebo or SBI at 2.5 g BID or 5 g BID for 4 weeks, followed by a 20-week placebo-free extension phase with SBI at either 2.5 or 5 g BID. Intestinal fatty acid binding protein (I-FABP), zonulin, flagellin, lipopolysaccharide (LPS) and LPS-binding protein, and inflammatory markers were measured by ELISA or multiplex assays. Non-parametric tests were used for analysis.
Results: One hundred three participants completed the study. By week 24 SBI significantly decreased circulating levels of I-FABP (-0.35 ng/mL, P = 0.002) and zonulin (-4.90 ng/mL, P = 0.003), suggesting improvement in gut damage, and interleukin-6 (IL-6) (-0.40 pg/mL, P = 0.002), reflecting improvement in systemic inflammation. In participants with the lowest quartile of CD4+ T-cell counts at baseline (189-418 cells/mL), CD4+ T-cell counts increased significantly (26 cells/mL; P = 0.002).
Conclusions: Oral SBI may decrease inflammation and warrants further exploration as a potential strategy to improve gut integrity and decrease systemic inflammation among persons receiving prolonged suppressive ART.
Keywords: HIV infection; CD4 T cell; Serum bovine immunoglobulin protein; Interleukin; Inflammation; Intestine; I-FABP
Clinical Trial Registry Number (Clinicaltrials.gov Identifier): NCT01828593
Human immunodeficiency virus (HIV) infection is characterized by profound depletion of CD4+ T cells systemically and in the gastrointestinal (GI) tract, compromised mucosal barrier function, translocation of microbial products, and chronic inflammation [1]. Suppressive antiretroviral therapy (ART) decreases but does not normalize inflammatory biomarkers [2, 3]. Consequently, diseases associated with increased inflammation are more common in people with HIV, including cardiovascular events, non-AIDS malignancies, liver disease, and others [4].
A key contributor to this chronic inflammation is increased translocation of microbial products across a permeable gut barrier from the intestinal lumen to the lamina propria and systemic circulation [1, 2]. These microbial products activate innate immune cells to produce pro-inflammatory cytokines, such as interleukin‑1β (IL‑1β), interleukin-6 (IL‑6), and tumor necrosis factor-α (TNF-α) [1]. Indeed, circulating markers of intestinal permeability (intestinal fatty acid binding protein [I-FABP] and zonulin) and IL-6 have been consistently predictive of non-AIDS events and mortality in people receiving suppressive ART [2, 5]. Thus, an intervention that decreases intestinal permeability and systemic inflammation, and its drivers, could decrease the excess morbidity and mortality associated with well-treated HIV infection.
Gut inflammatory markers are decreased in animal models of colitis after oral administration of plasma protein preparations with high levels of immunoglobulins [6, 7]. Serum-derived bovine immunoglobulin/protein isolate (SBI) is an oral powder containing high concentrations of immunoglobulin that is not absorbed. It is the primary ingredient in a medical food that has been used successfully to manage patients with diarrhea-predominant irritable bowel syndrome (IBS-D) and inflammatory bowel disease [8-10]. SBI is enriched for immunoglobulins, comprised of > 50% IgG, 1% IgA, and 5% IgM, which bind lipopolysaccharide (LPS) or endotoxin and other conserved microbial antigens [11-13], in addition to amino acids, albumin, and transferrin [14]. In a previous study involving 8 patients with HIV-associated diarrhea, we found improvements in GI symptoms, lymphocyte populations in gut-associated lymphoid tissue (GALT), and markers of intestinal repair following 8 weeks of SBI therapy [15]. In a follow up study, we found improvements in stool frequency and several other GI symptoms but with a greater than expected placebo effect [16]. Here, we aim to report the impact of oral SBI on biomarkers of intestinal integrity and systemic inflammation in virologically suppressed persons with HIV with gastrointestinal symptoms.
Adult men or non-pregnant women with HIV and virologic suppression for at least 12 months were eligible if they had enteropathy; this was defined as 3 or more loose stools per day for at least 3 months. Participants were not eligible to participate if they had (1) a positive stool test for pathogenic bacteria, ova, or parasites during the 14-day screening period, (2) changes in antiretroviral medications during the 3-month period prior to screening, or (3) a condition that required chronic therapy that might alter the gut flora or the use of an antibiotic within 2 weeks prior to screening. Institutional Review Boards at each site reviewed and approved the protocol [Clinical Trial Registry Number Identifier (Clinicaltrials.gov): NCT01828593], and all participants provided written informed consent.
This prospective, multicenter, randomized, blinded study included a partial cross-over design comprising 2 study phases: a double-blind placebo (PBO)-controlled phase and a placebo-free blinded extension phase. Participants were randomized at study entry to (1) placebo BID for 4 weeks followed by either twice daily LD-SBI (low dose-SBI, 2.5 g) for 20 weeks (n = 21) or HD-SBI (High dose-SBI, 5 g) for 20 weeks (n = 14); (2) Twice daily LD-SBI for 24 weeks (n = 34); or (3) Twice daily HD-SBI for 24 weeks (n = 33; Supplementary Figure 1). Participants received placebo (2.5 g dextrose) or SBI dissolved in 120 mL of water and remained blinded to their treatment assignment–whether they had received the placebo lead-in or low dose versus high dose assignment for the duration of the study. Thirteen of 103 randomized participants did not complete the study as previously described [16]. All study participants had blood samples obtained at baseline and weeks 4, 8, and 24 to evaluate PBMCs and biomarkers of bacterial translocation, inflammation, enterocyte damage, coagulation, and pro-inflammatory cytokines.

The primary efficacy endpoint of the parent clinical trial was the change in number of bowel movements (BM) per day from baseline to week 4, as previously reported [16]. Predetermined exploratory endpoints included changes in peripheral CD4+and CD8+ T-cell counts, plasma markers of intestinal damage, microbial translocation, inflammation, and coagulation at week 4 and last observation. Peripheral CD4+ and CD8+ T-cell counts were measured by flow cytometry using standardized methods (North Coast Clinical Laboratory, Inc., Sandusky, OH).
The level of LPS was assayed in duplicate using a Lonza LAL QCL-1000 kit (Lonza, Walkersville, MD) according to the manufacturer’s protocol. LPS, LPS-binding protein (LBP), and Bactericidal/Permeability Increasing Protein (BPI) were measured in plasma samples using enzyme-linked immunosorbent assay (ELISA)-based assays according to the manufacturer's protocol (MyBioSource, Inc., San Diego, CA), and flagellin was measured by ELISA in serum (USCN Life Science Inc., Missouri City, TX).
Levels of pro-inflammatory cytokines (IL-1β, TNF-α), Th1 (interferon-γ [IFN-γ] and IL-12p70), Th2 (IL-4), and regulatory cytokines (IL-10), chemokines (IL-8), and monocyte chemoattractant protein-1 (MCP-1 or CCL2), and biomarkers of collagen regulation (hyaluronic acid (HA), transforming growth factor-β (TGF-β) 1, 2, and 3, matrix metalloproteinases (MMP)-1, MMP-9, tissue inhibitor of metalloproteinases (TIMP-1, and TIMP-2) were all measured in plasma with multiplex ELISA-based assays (MesoScale Discovery; assay kit for HA from Corgenix, Broomfield, CO). Serum levels of IL-6 and soluble CD14 (sCD14) were measured with Magnetic Luminex® Kits (R&D Systems, Minneapolis, MN).
Serum intestinal fatty acid binding protein (I-FABP) is an intestinal isoform of a ubiquitous intracellular protein that is known to be elevated in diseases of chronic intestinal inflammation such as celiac disease as well as in people with HIV and chronic diarrhea. I-FABP was measured using an ELISA–based assay (R&D Systems, Minneapolis, MN). Serum zonulin levels were measured using a competitive ELISA method (Immundiagnostik AG, Bensheim, Germany).
The analysis used change from baseline measures to the end of week 4 of the PBO-controlled phase of the study and change from baseline to last observation carried forward (LOCF) in the 20-week PBO-free phase of the study from all available double-blind data without imputation of missing data. LOCF data encompasses participants who received SBI for 24 weeks (LD-SBI and HD-SBI) or 20 weeks (PBO crossover at Week 4). A 2-sided significance test using α of 0.05 was used to declare statistical significance and flag results for further inquiry. Changes from baseline data were analyzed using Wilcoxon signed-rank tests of the paired samples. As these analyses were exploratory, no corrections for multiplicity were implemented, and all significant results are considered hypothesis generating [17]. All statistical analyses were performed with SAS software version 9.3 (SAS Institute Inc, Cary, NC).
Characteristics of the participants at study entry have been described previously and are summarized in Table 1 [16]. The median age was 50 years, with 69% male, 61% African-American and 37% White participants. Median time since HIV diagnosis was 18.2 years, with a median duration of ART of 8.33 years and duration of HIV-associated diarrhea of 3.5 years. Overall, median HIV-1 RNA level was undetectable (defined as 19 copies/mL) across all arms, and CD4+ T-cell count was 637 cells/mm3, with no difference between arms (PBO–median 523 cells/mL [Q1 397, Q3 921], LD-SBI–813 cells/mL [469, 939] and HD-SBI–672 cells/mL [478, 776]). The distribution at baseline in the top 3 CD4+ T-cell quartiles (> 418 cells/mL) was near or in the normal range for people taking chronic suppressive ART.
Table 1. Baseline Characteristics for Randomized Treatment Groups
|
|
n (%) or median (Q1-Q3) |
||||
|
Characteristic |
Placebo |
SBI 2.5 g |
SBI 5.0 g |
Total |
|
|
Study Subjects a |
36 |
34 |
33 |
103 |
|
|
Sex |
Male |
28 (78) |
21 (62) |
22 (67) |
71 (69) |
|
Female |
8 (22) |
13 (38) |
11 (33) |
32 (31) |
|
|
Race |
African American |
21 (58) |
20 (59) |
22 (67) |
63 (61) |
|
White |
14 (39) |
14 (41) |
10 (30) |
38 (37) |
|
|
Asian |
1 (3) |
0 (0) |
1 (3) |
2 (2) |
|
|
Age (years) |
50 (34–66) |
49 (34–70) |
48 (32–65) |
50 (32–70) |
|
|
Time since HIV diagnosis (years) |
16.7 (1.8–27.5) |
16.4 (6.3–29.5) |
19.2 (4.8–28.5) |
18.2 (1.8–29.5) |
|
|
Peripheral CD4+ T-cell count (cells/mL) b |
523 (194–1224) |
813 (189–1611) |
672 (202–1754) |
637 (189–1754) |
|
|
Plasma Viral Load (copies/mL) c |
19 (19–64) |
19 (19–119) |
19 (19–168) |
19 (19–168) |
|
|
Time on ART (years) |
7.46 (1.0–23.07) |
9.05 (1.0–23.67) |
9.89 (1.0–23.73) |
8.33 (1.0–23.73) |
|
|
Time with HIV-associated Diarrhea (years), median (Q1-Q3) |
2.2 (0.2–23.7) |
4.7 (0.2–29.5) |
5.5 (0.1–23.5) |
3.5 (0.1–29.5) |
|
a Analysis population = all randomized participants receiving at least 1 dose of investigational product during placebo-controlled phase.
b Quartile ranges were as follows: Q1 :≤418; Q2: > 418 to ≤ 630; Q3: > 630 to ≤ 893; Q4: > 893
c Limit of detection for plasma viral load was 20 copies/mL
Abbreviations: SBI, Serum-derived bovine immunoglobulin/protein isolate; ART, antiretroviral therapy
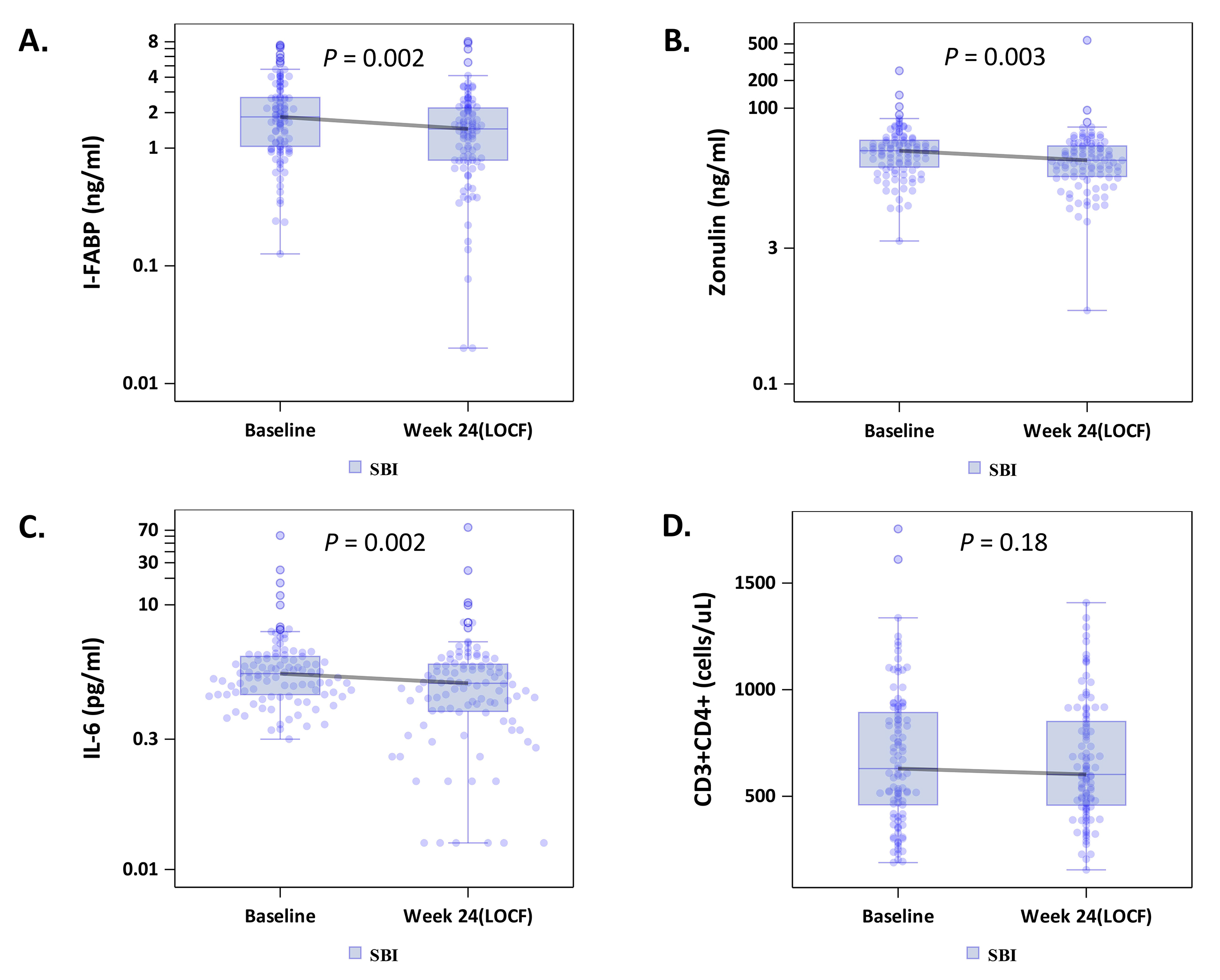
To explore whether SBI administration would improve gut barrier function, we measured circulating I-FABP levels, reflecting enterocyte turnover [18], and zonulin levels, reflecting tight junction integrity [19]. Median levels of both I-FABP, -0.35 ng/mL (-1.46, +0.31; P = 0.002), and zonulin, -4.90 ng/ml (-18.43, +4.51; P = 0.003), decreased in the combined SBI groups (LD-SBI and HD-SBI, n = 100) (Table 2; Figures 1A and B); changes within treatment groups at week 4 were not significant (Supplementary Table 1). I-FABP and zonulin levels decreased in both SBI dose groups, with a trending dose effect (Table 3). Circulating markers of microbial translocation, however, including LPS, LBP, 16S rDNA, flagellin, and sCD14 (Tables 2 and 3 and not shown) did not change significantly with SBI therapy. As lower CD4+ T-cell counts are associated with more gut damage and microbial translocation [2], we evaluated the lowest baseline CD4+ T-cell quartile (189-418 cells/mL; n = 25) separately. I-FABP, zonulin, and flagellin changes decreased without statistical significance in this group, although the small sample size might have limited the power to detect a change (Table 4; Figure 2A-B).
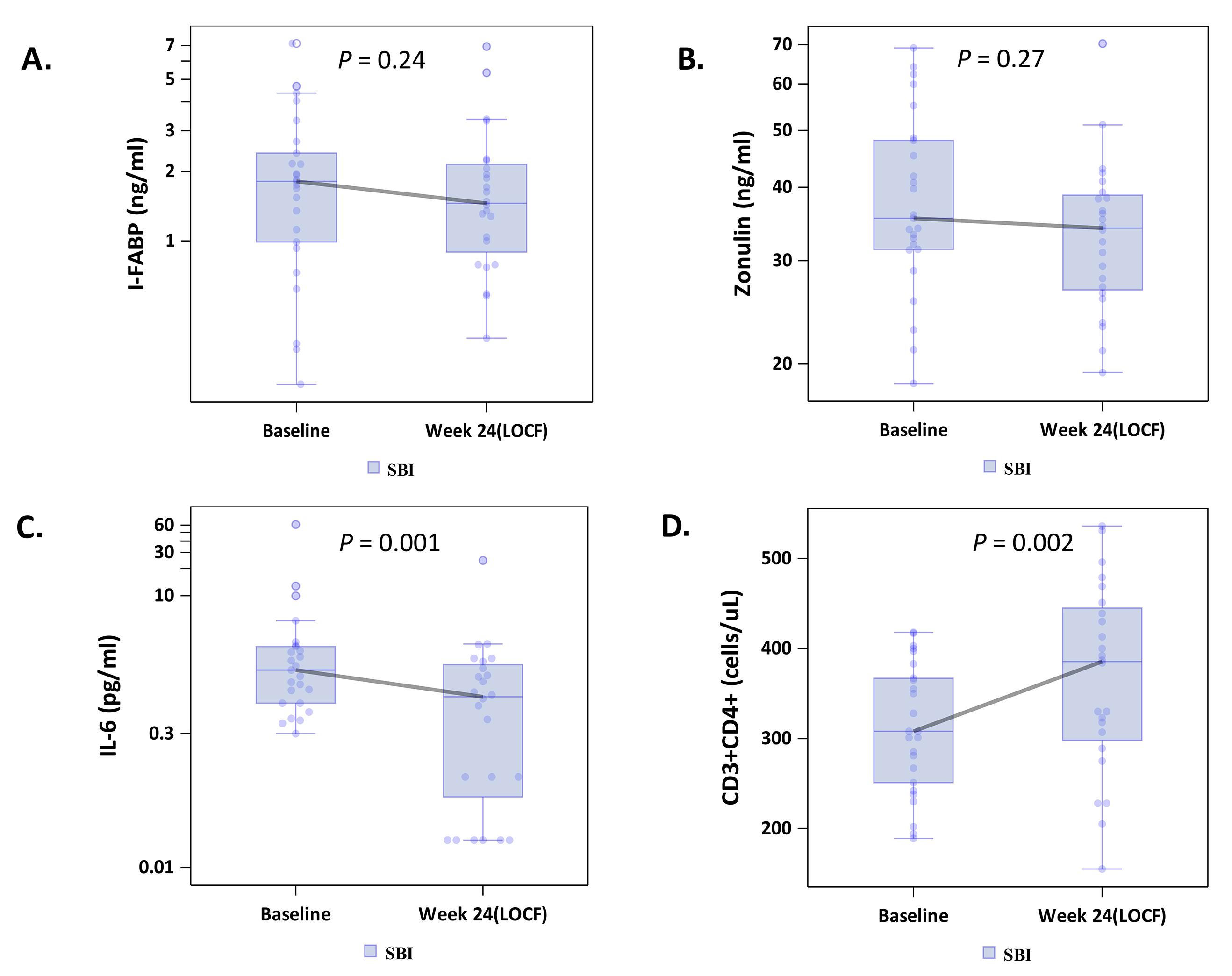
Figure 2. Effects of serum bovine immunoglobulin/protein isolate (SBI) on biomarkers of intestinal permeability (A-B; intestinal fatty acid binding protein [I-FABP], zonulin), (C) inflammation (IL-6) and (D) CD4+ T-cell counts among participants with the lowest quartile of CD4+ T-cell counts.
Table 2. Within-Group Comparison for Combined SBI Groupsa
|
Variable |
Treatment
|
n Median (Q1-Q3) |
P- valued |
|||||
|
Baseline |
LOCFc |
Delta |
||||||
|
CD4 (cells/uL) |
SBI |
101 |
630 (460–893) |
100 |
602.50 (458.50–851) |
99 |
-14 (-119–80) |
0.177 |
|
CD4/CD8 |
SBI |
101 |
0.71 (0.47–1.17) |
100 |
0.72 (0.49–1.17) |
99 |
0 (-0.07–0.07) |
0.997 |
|
|
||||||||
|
I-FABP (ng/mL) |
SBI |
101 |
1.84 (1.03–2.69) |
100 |
1.46 (0.79–2.19) |
99 |
-0.35 (-1.46–0.31) |
0.002 |
|
|
||||||||
|
Zonulin (ng/mL) |
SBI |
101 |
34.45 (22.87–44.74) |
100 |
27.09 (18.04–38.79) |
99 |
-4.90 (-18.43–4.51) |
0.003 |
|
|
||||||||
|
Flagellin (ng/mL) |
SBI |
101 |
4.76 (2.97–7.23) |
100 |
4.62 (2.52–7.20) |
99 |
-0.18 (-2.66–1.76) |
0.360 |
|
|
||||||||
|
sCD14 (ng/mL) |
SBI |
101 |
1.96 (1.62–2.23) |
100 |
1.89 (1.56–2.16) |
99 |
-0.03 (-0.28–0.24) |
0.375 |
|
|
||||||||
|
IL-6 (pg/mL) |
SBI |
101 |
1.66 (0.96–2.60) |
100 |
1.29 (0.62–2.13) |
99 |
-0.40 (-1.25–0.35) |
0.002 |
|
|
||||||||
a Analysis population = Participants receiving SBI for 24 weeks and participants crossing-over from PBO to SBI after week 4
b Combined SBI treatment groups
c Last observation carried forward, to 24 weeks for SBI-treated group and to 20 weeks for PBO-SBI cross-over group
d Wilcoxon Signed-Rank test
Abbreviations: SBI, Serum-derived bovine immunoglobulin/protein isolate; LOCF, Last observed carried forward; I-FABP, intestinal fatty acid binding protein; sCD14, soluble CD14
Table 3. Within-Group Comparison for SBI Groupsa
|
Variable |
Treatment
|
n Median (Q1-Q3) |
P- valuec |
|||||
|
Baseline |
LOCFb |
Delta |
||||||
|
CD4 (cells/uL) |
SBI 2.5 g |
54 |
803 (469–958) |
55 |
638 (466–915) |
54 |
-38 (-158–67) |
0.018 |
|
SBI 5.0 g |
47 |
543 (757–417) |
45 |
567 (413–800) |
45 |
16 (-67–83) |
0.539 |
|
|
|
||||||||
|
CD4/CD8 |
SBI 2.5 g |
54 |
0.76 (0.46–1.42) |
55 |
0.81 (0.47–1.35) |
54 |
0 (-0.06–0.08) |
0.772 |
|
SBI 5.0 g |
47 |
0.66 (1–0.48) |
45 |
0.65 (0.51–0.98) |
45 |
0.01 (-0.07–0.06) |
0.760 |
|
|
|
||||||||
|
I-FABP (ng/mL) |
SBI 2.5 g |
54 |
1.95 (0.99–3.11) |
55 |
1.40 (0.79–2.24) |
54 |
-0.23 (-1.48–0.24) |
0.023 |
|
SBI 5.0 g |
47 |
1.81 (1.10–2.66) |
45 |
1.56 (1–2.10) |
45 |
-0.45 (-1.17–0.36) |
0.043 |
|
|
|
||||||||
|
Zonulin (ng/mL) |
SBI 2.5 g |
54 |
34.61 (19.43–42.24) |
55 |
29.25 (20.59–38.24) |
54 |
-4.16 (-17.07–6.85) |
0.117 |
|
SBI 5.0 g |
47 |
34.27 (24.16–49.35) |
45 |
26.42 (17.33–40.95) |
45 |
-6.18 (-21.44–3.03) |
0.009 |
|
|
|
||||||||
|
Flagellin (ng/mL) |
SBI 2.5 g |
54 |
4.34 (2.55–7.16) |
55 |
4.22 (1.83–6.61) |
54 |
-0.22 (-2.61–1.55) |
0.307 |
|
SBI 5.0 g |
47 |
5.14 (3.15–8.02) |
45 |
5.36 (3.42–7.87) |
45 |
-0.07 (-2.66–1.76) |
0.792 |
|
|
|
||||||||
|
sCD14 (ng/mL) |
SBI 2.5 g |
54 |
2.02 (1.70–2.27) |
55 |
1.99 (1.69–2.20) |
54 |
-0.08 (-0.29–0.26) |
0.433 |
|
SBI 5.0 g |
47 |
1.87 (1.41–2.20) |
45 |
1.79 (1.43–2.05) |
45 |
0 (-0.28–0.21) |
0.675 |
|
|
|
||||||||
|
IL-6 (pg/mL) |
SBI 2.5 g |
54 |
1.72 (1.20–2.71) |
55 |
1.27 (0.65–2.07) |
54 |
-0.53 (-1.27–0.25) |
0.012 |
|
SBI 5.0 g |
47 |
1.37 (0.91–2.46) |
45 |
1.31 (0.60–2.18) |
45 |
-0.38 (-1.13–0.40) |
0.072 |
|
|
|
||||||||
a Analysis population = Patients receiving SBI for 24 weeks and patients crossing-over from PBO to SBI after week 4
b Last observation carried forward, to 24 weeks for SBI-treated group and to 20 weeks for PBO-SBI cross-over group
c Wilcoxon Signed-Rank test
Abbreviations: SBI, Serum-derived bovine immunoglobulin/protein isolate; LOCF, Last observed carried forward; I-FABP, intestinal fatty acid binding protein; sCD14, soluble CD14
Table 4. Within-Group Comparison for Combined SBI Groupsa in the first CD4+ T-cell Quartileb.
|
Variable |
Treatment |
n Median (Q1-Q3) |
P- valued |
|||||
|
Baseline |
LOCFc |
Delta |
||||||
|
CD4 (cells/uL) |
SBI |
25 |
308 (251–367) |
24 |
385.50 (298–445) |
24 |
25.50 (-0.50–125) |
0.002 |
|
|
||||||||
|
CD4/CD8 |
SBI |
25 |
0.38 (0.24–0.53) |
24 |
0.33 (0.27–0.57) |
24 |
0.01 (-0.06–0.06) |
0.848 |
|
|
||||||||
|
I-FABP (ng/mL) |
SBI |
25 |
1.81 (0.99–2.40) |
24 |
1.46 (0.90–2.15) |
24 |
-0.35 (-0.78–0.30) |
0.244 |
|
|
||||||||
|
Zonulin (ng/mL) |
SBI |
25 |
35.42 (31.35–48.07) |
24 |
34.08 (26.74–38.79) |
24 |
-1.93 (-15.89–4.63) |
0.274 |
|
|
||||||||
|
Flagellin (ng/mL) |
SBI |
25 |
5.04 (2.53–7.17) |
24 |
4.78 (3.04–5.86) |
24 |
-0.57 (-2.06–0.94) |
0.122 |
|
|
||||||||
|
sCD14 (ng/mL) |
SBI |
25 |
1.78 (1.40–2) |
24 |
1.69 (1.40–1.94) |
24 |
0.01 (-0.30–0.25) |
0.825 |
|
|
||||||||
|
IL-6 (pg/mL) |
SBI |
25 |
1.51 (0.65–2.74) |
24 |
0.77 (0.06–1.73) |
24 |
-0.57 (-1.26–-0.16) |
0.001 |
|
|
||||||||
a Analysis population = Participants receiving SBI for 24 weeks and participants crossing-over from PBO to SBI after week 4
b
Analysis subpopulation = participants in first CD4+T-cell
quartile ranges were as follows: Q1 :≤418 (cell/ uL) at baseline. Q2-Q4
are: > 418 to ≤ 630,
> 630 to ≤ 893, and > 893, respectively
c Last observation carried forward, to 24 weeks for SBI-treated group and to 20 weeks for PBO-SBI cross-over group
d Wilcoxon Signed-Rank test.
Abbreviations: SBI, Serum-derived bovine immunoglobulin/protein isolate; LOCF, Last observed carried forward; I-FABP, intestinal fatty acid binding protein; sCD14, soluble CD14
Because IL-6 is a marker of inflammation that correlates with clinical outcomes [5], we measured the impact of SBI therapy on IL-6 levels. The median serum IL-6 level among all participants decreased significantly from baseline to last observation carried forward by -0.40 pg/mL (-1.25, +0.35; P = 0.002, n = 100) (Table 2; Figure 1C), with no obvious dose effect (Table 3). Notably, 83% of people in the lowest baseline CD4+ quartile had decreases in serum IL-6 levels, with a median change among all participants in this quartile of -0.57 pg/mL (-1.26, -0.10; P = 0.001, n = 24; Table 4; Figure 2C).
Among the combined SBI groups, peripheral CD4+ T-cell counts did not change (Table 2 and Figure 1D). Peripheral CD4+ T-cell counts decreased in participants who received LD-SBI from 803 to 638 cells/mL (-38 cells/mL [-158, +67], P = 0.018; n = 54), but not in those receiving HD-SBI (+16 cells/mL [-67, +83], P = 0.539; n = 45) between baseline and last observation carried forward (Table 3). Changes in CD8+ T cells correlated with changes in CD4+ T cells (r = 0.74, P < 0.0001; data not shown). Consequently, no significant changes were observed in CD4/CD8 T-cell ratios from baseline to week 24 for any of the treatment groups (Table 3).
We examined changes in CD4+ T-cell counts in the lowest quartile of CD4+ T-cell counts because people who have failed to normalize CD4+ T-cell counts may have the greatest potential to experience an immunologic benefit from attenuating systemic inflammation. Among participants in the lowest baseline CD4+ T-cell quartile (189-418 cells/mL; n = 25), peripheral CD4+ T-cell counts for the combined SBI groups (LD-SBI+HD-SBI) increased from baseline (308 cells/mL) to last observation carried forward (386 cells/mL) after 20-24 weeks of SBI, with a median change of 25.5 cells/mL (-0.5, +125; P = 0.002) (Table 4; Figure 2D); 75% of participants had an increase in CD4+ T-cell counts. This increase was paralleled by an increase in CD8+ T-cell counts of 109 cells/mL (+8, +280; P = 0.004; data not shown), from 806 to 964 cells/mL, with 79% of participants having an increase in CD8+ T-cell counts. The CD4/CD8 ratio, however, did not change significantly from baseline (0.38) to last observation (0.33), with a median change of 0.01 (-0.06, +0.06; P = 0.85; Table 4). Thus, CD4+ and CD8+ T-cell counts increased significantly during SBI treatment in participants with the lowest CD4+ T-cell counts.
Next, we evaluated associations between changes in biomarker levels (I-FABP, zonulin, IL-6, sCD14) and immunologic and clinical parameters from baseline to last observed measurement using Spearman correlations. Changes in flagellin (r = -0.40, P < 0.0001), zonulin (r = -0.62, P < 0.0001), I-FABP (r = -0.65, P < 0. 0001), and IL-6 (r = -0.56, P < 0.0001) correlated with baseline levels of each respective biomarker, and changes in serum I-FABP levels were associated inversely with changes in CD4/CD8 ratios among all participants (r = -0.307, P = 0.003; n = 100) (Figure 3). Changes among biomarkers of gut damage did not correlate with changes in biomarkers of inflammation. Participants who had decreases in sCD14 levels tended to be younger (r = 0.21, P = 0.04) and to have decreases in the frequency of loose stool (P = 0.06). We saw no associations between changes in other biomarkers or CD4+ T-cell count and age or frequency of stools. For the lowest CD4+ T-cell quartile group, decreases in IL-6 levels were associated with decreases in sCD14 levels (r = 0.535, P = 0.008; n = 24). In addition, for the lowest CD4+ T-cell quartile group, lower IL-6 levels at week 24 were associated with higher CD4/CD8 ratios (r = -0.516, P = 0.012). Thus, I-FABP, zonulin, and IL-6 levels decreased with SBI, and decreases in markers of intestinal permeability and inflammation correlated with increases in CD4/CD8 ratios.
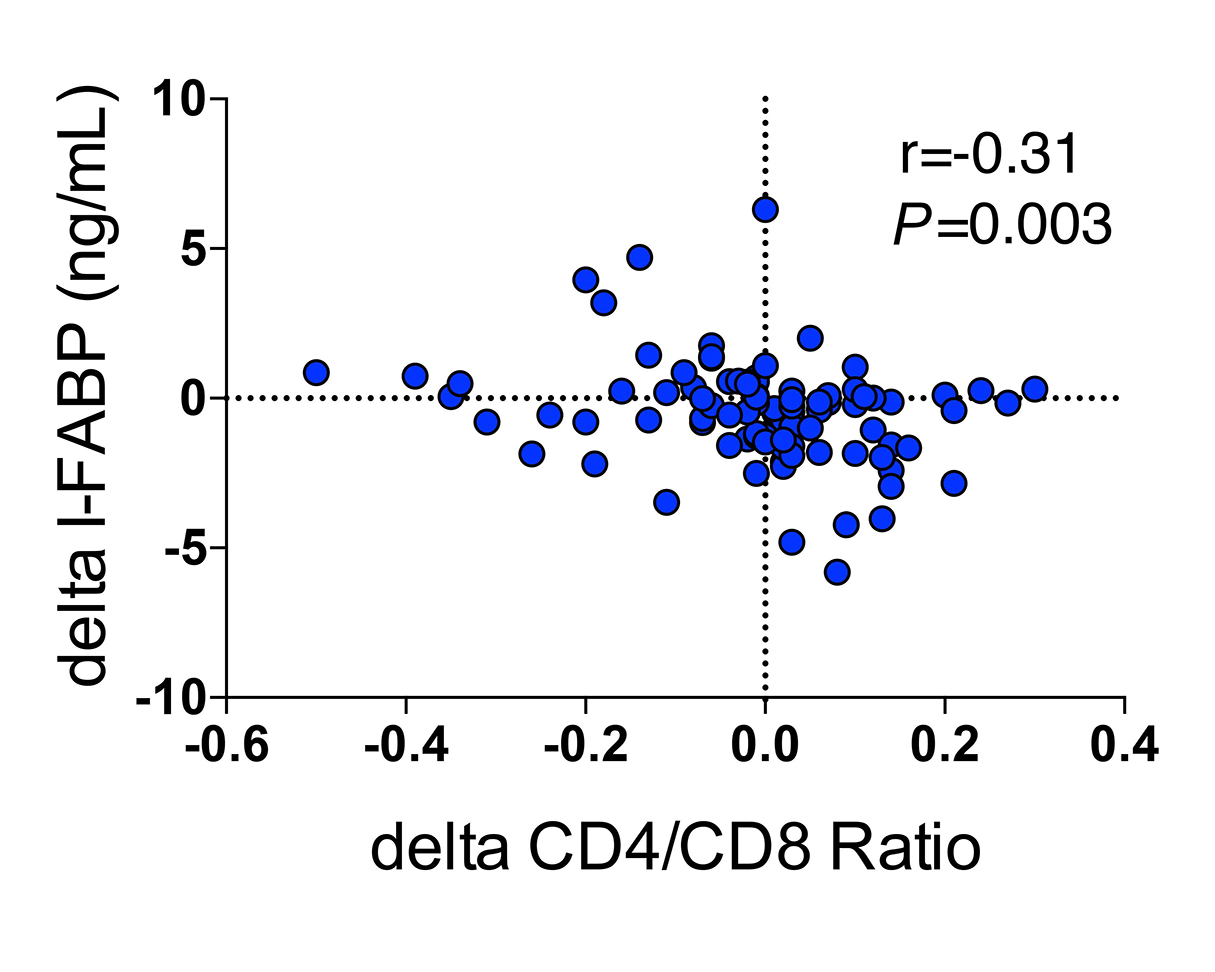
Figure 3. Association of changes in I-FABP with changes in CD4/CD8 T-cell ratios.
We previously showed that SBI is safe and well-tolerated in people with HIV receiving ART and with chronic diarrhea [16]. People treated with SBI had decreased diarrhea and other GI symptoms but without significant differences from the placebo group. Here, we explored the effects of SBI on biomarkers of gut damage, microbial translocation, and inflammation, and on CD4+ T-cell recovery, as predefined exploratory endpoints. We found that SBI treatment was associated with 1) decreases in I-FABP and zonulin levels, 2) decreases in IL-6 levels, particularly among those with the lowest baseline CD4+ T-cell counts, and 3) increases in CD4+ and CD8+ T-cell counts among participants with the lowest baseline CD4+ T-cell counts. Together, these findings suggest that SBI warrants further exploration as a potential intervention to improve gut integrity and decrease inflammation.
SBI likely works by multiple mechanisms. SBI may bind luminal microbial antigens, preventing their translocation into the lamina propria via steric exclusion and preventing their ability to activate macrophages and dendritic cells [13, 14]. Indeed, SBI can bind bacterial, viral, and fungal microbe-associated molecular patterns. The ensuing lack of antigenic stimulation may explain why SBI has been shown to decrease leukocyte recruitment into the lamina propria [20]. Much like with intravenous immunoglobulin, binding of the Fc portion of IgG in SBI to Fc receptors on target T cells may enhance its anti-inflammatory effect [14]. SBI has also been shown to increase the abundance of Proteobacteria Burkholderiales, Firmicutes Catonella, and other bacteria in the small intestine [21] that may be associated with improvement in gut health [22]. A similar intervention, purified protein concentrate from plasma, has been shown to increase tight junction formation in weaning piglets and in a rat model of intestinal colitis [14]. Amino acids in SBI such as glutamine may further facilitate epithelial healing [14]. Whether growth factors are present in SBI that could stimulate T-cell proliferation, accounting for the increase in CD4+ T cells, warrants future determination. Thus, use of bioactive proteins as found in SBI may improve intestinal epithelial barrier function through immunomodulation, alteration of gut bacteria, and increasing tight junction formation [23].
Zonulin opens tight junctions reversibly in response to small intestinal exposure to enteric bacteria such as E. coli [24]. The small intestine has a low bacterial burden (up to 104 colony forming units [CFU]/mL) in healthy people, predominantly Gram-positive aerobic bacteria proximally and facultative anaerobes distally, whereas the colon contains 1012 CFU/mL of strict anaerobes [25]. Small intestinal colonization by colonic flora upregulates zonulin and consequently increases intestinal permeability [25]. Lower zonulin levels have been associated with more bacterial diversity, suggesting the composition of the small intestinal bacteria impacts zonulin levels [26]. Zonulin levels are higher in Crohn's disease [27] and experimental cholera [28], and other intestinal diseases, indicating that in most circumstances, high zonulin levels reflect intestinal disease. Unexpectedly, in a previous study of people with HIV, higher zonulin levels were associated with lower mortality risk in a population with low current and nadir CD4+ T-cell counts (median nadir 30 cells/mm3) [2]. The high zonulin levels in survivors in this study may reflect the preservation of cells capable of producing zonulin and therefore better gut health rather than increased tight junction damage. In contrast, our population had higher CD4+ T-cell counts and normal nutrient absorption; therefore, the decrease in zonulin levels we observed may reflect a decrease in stimulation, such as dysbiosis and bacterial overgrowth, rather than a decrease in the cells capable of producing the protein. The decrease in I-FABP levels, which has been associated with decreased mortality, [2] reflects decreased enterocyte turnover, consistent with our hypothesis and previous findings [15]. Levels of I-FABP increase from acute to chronic HIV infection and, in contrast to many biomarkers predictive of poor outcomes, increase further with ART [29]. The simultaneous decrease in I-FABP and zonulin suggests that SBI decreases enterocyte turnover. Whether this reflects an improvement in gut health is unclear, but the simultaneous decrease in IL-6 indicates it may be beneficial.
Contrary to our hypothesis, no direct marker of microbial translocation, including LPS, flagellin, and 16S rDNA, was affected by SBI treatment. Plasma markers of microbial translocation decrease with long-term ART [3], so it is possible that our study was not sufficiently powered to detect further reductions in these markers. As improved gut health would need to precede changes in microbial translocation, this study may have been too short to detect decreases in these circulating markers. Moreover, several factors are known to interfere with LPS measurement [1], leading to the suggestion that indicators of the host response to LPS may be more accurate and relevant than measurement of LPS itself. The lack of effect on sCD14 levels, one such indicator, suggests that prevention of microbial translocation may not be the primary mechanism by which SBI can decrease inflammation.
The decrease in IL-6 levels observed in HIV participants following 24 weeks of oral SBI, accompanied by decreases in I-FABP and zonulin but not microbial translocation markers, suggests that 1) the upstream mediator of gut damage also upregulates IL-6 production, 2) increased IL-6 production may disrupt the gut barrier, or 3) increased gut barrier dysfunction stimulates IL-6 production. Whether these findings are due to alterations in the gut microbiome that facilitate epithelial healing and decrease inflammation, immunomodulatory proteins in SBI, or decreased translocation of microbial products not detected in the plasma is unknown. Some studies have suggested immune activation and inflammation improve during clinical studies due to increased participant adherence. [30-32] Excellent self-reported adherence including at entry and persistently undetectable HIV-1 RNA levels suggest that improved adherence is unlikely to contribute to the decreased inflammation observed here, although we did not measure HIV-1 RNA by single-copy assay. Assuming that the decrease in IL-6 is due to SBI and given that higher IL-6 levels are associated with increased atherosclerosis and cardiovascular disease [33,34], AIDS events [35], non-AIDS events [36], and mortality [2,34, 37], an intervention that decreases its levels could have a profound impact on clinical outcomes in HIV infection. We demonstrated a 38% decrease in IL-6 levels in participants in the first quartile; IL-6 levels were about 40% higher in participants who died compared to controls in the SMART study [38] and about 40% higher in immune non-responders compared to immune responders [39], suggesting the difference observed here may be clinically relevant. Further investigations into the mechanism of action may highlight ways to improve SBI's efficacy and potentially ways to synergize with other interventions.
The increase in CD4+ T cells in participants with the lowest quartile of CD4+ T cells at baseline is unlikely to reflect increased adherence as noted above [31]. One possibility is that this increase is due to decreased immune activation by SBI. Immune activation has been associated with poor CD4+ T-cell recovery [2], and immunosuppression with prednisolone in the absence of ART can improve CD4+ T-cell counts [40]. SBI may decrease apoptosis of activated CD4+ T cells [41] or downregulate adhesion molecules on the surface of CD4+ T cells [41], allowing egress of CD4+ T cells from tissues into the circulation [42]. Alternatively, immunoglobulins or other factors in SBI may increase de novo thymic output of CD4+ cells or expansion of CD4+ T-cell populations [43]. The parallel findings in CD8+ T cells suggest that the mechanism is not CD4+ T-cell specific, and it is not likely to be mediated by changes in residual HIV replication. These findings, however, could reflect regression to the mean, and the changes observed may not be due to any effect of SBI. Evaluating SBI in a longer, randomized, placebo-controlled trial in immunologic non-responders is essential for determining whether it has any effect on CD4+ T-cell recovery.
Other agents have been tested to improve gut integrity and decrease microbial translocation and inflammation in chronic HIV infection. Most agents targeting gut health had little if any effect, including sevelamer carbonate [44], rifaximin [45], mesalamine [46], and prebiotics and probiotics [47]. Immunomodulatory agents such as atorvastatin [48] have also been disappointing. Notably, hydroxychloroquine decreased IL-6 and LPS levels in immunologic non-responders [49], corticosteroids decreased IL-6 in viremic participants receiving ART [50] and sCD14 in untreated participants [40], and rosuvastatin, in contrast to atorvastatin, decreased sCD14 levels [51]. Recently, canakinumab, an IL-1b antibody, was shown to decrease IL-6 levels in a pilot study of people receiving suppressive ART [52]. However, these drugs operate systemically with pleiotropic effects and are not without the potential for serious adverse events. Gut-targeted treatment with oral SBI might be a safer means to decrease inflammation.
This study had several limitations. First, there was no placebo arm for the entire duration of the study. Thus, it is possible that the changes observed at 24 weeks could be due to continued ART alone, although previous literature suggests stability of these biomarkers given the duration of ART [3,45, 46], and this population had a median duration of ART of > 8 years. Second, the increases in peripheral CD4+ T-cell counts were observed during post hoc analysis and may reflect regression to the mean. Therefore, these results need to be interpreted cautiously. Third, study participants had a wide range of duration of virologic suppression (1-23 years), which could have influenced the changes observed [41]. Fourth, participants spanned a wide age range (32 to 70 years); older individuals have more immune activation [53] and are at higher risk for persistently reduced CD4+ T-cell counts during ART [54]. Lastly, numerous correlations were assessed and no adjustments were performed, which has been supported for exploratory analyses [17], but it is possible the positive findings could reflect noise rather than true biological associations.
In conclusion, our results demonstrate that oral administration of SBI improved markers of gut barrier function and systemic inflammation. A longer, randomized, placebo-controlled trial of SBI in people with HIV and immunologic failure is needed to assess the clinical impact and mechanisms of action of SBI.
We are deeply indebted to the generosity and willingness of the volunteers, without whom this research would be impossible. We would like to thank the nurses and support personnel in the University of California CTSC Clinical Research Center, the Veterans Administration Hospital, and University of California Gastroenterology Suites for their dedication and assistance in this research effort.
We thank faculty and nursing staff of the Center for AIDS Research, Education, and Services clinic for help in study participant recruitment.
This research was made possible by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health, and NIH Roadmap for Medical Research. The clinical trial was supported by Entera Health, Inc. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Entera Health, Inc.
Christopher Detzel is a current employee of Entera Health, Inc. Bryon Petschow, Audrey Shaw, and Eric Weaver are all former employees of Entera Health, Inc. All remaining authors declare no conflicts of interest.
1. Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655-66. PubMed PMID: 22886237. doi: 10.1038/nrmicro2848
2. Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, Deeks SG, Meinert CL, Van Natta ML, Jabs DA, Lederman MM. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210(8):1228-38. PubMed PMID: 24755434. Pubmed Central PMCID: PMC4192038. doi: 10.1093/infdis/jiu2383. Funderburg NT, Andrade A, Chan ES, Rosenkranz SL, Lu D, Clagett B, Pilch-Cooper HA, Rodriguez B, Feinberg J, Daar E, Mellors J, Kuritzkes D, Jacobson JM, Lederman MM. Dynamics of immune reconstitution and activation markers in HIV+ treatment-naive patients treated with raltegravir, tenofovir disoproxil fumarate and emtricitabine. PLoS One. 2013;8(12):e83514. PubMed PMID: 24367599. Pubmed Central PMCID: Pmc3867440. doi: 10.1371/journal.pone.0083514
4. Rasmussen LD, May MT, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, Obel N. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV. 2015;2(7):e288-98. PubMed PMID: 26423253. doi: 10.1016/s2352-3018(15)00077-6
5. Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis. 2014;210(8):1248-59. PubMed PMID: 24795473. Pubmed Central PMCID: Pmc4192039. doi: 10.1093/infdis/jiu254
6. Perez-Bosque A, Miro L, Maijo M, Polo J, Campbell J, Russell L, Crenshaw J, Weaver E, Moreto M. Dietary intervention with serum-derived bovine immunoglobulins protects barrier function in a mouse model of colitis. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2015;308(12):G1012-8. PubMed PMID: 25882614. doi: 10.1152/ajpgi.00378.2014
7. Henderson AL, Brand MW, Darling RJ, Maas KJ, Detzel CJ, Hostetter J, Wannemuehler MJ, Weaver EM. Attenuation of Colitis by Serum-Derived Bovine Immunoglobulin/Protein Isolate in a Defined Microbiota Mouse Model. Digestive Diseases and Sciences. 2015;60(11):3293-303. PubMed PMID: 26026602. Pubmed Central PMCID: PMC4621698. doi: 10.1007/s10620-015-3726-5
8. Wilson D, Evans MD, Weaver E, Shaw AL, Klein GL. Evaluation of Serum-Derived Bovine Immunoglobulin Protein Isolate in Subjects with Diarrhea-Predominant Irritable Bowel Syndrome. Clinical Medicine Insights: Gastroenterology. 2013;6:49-60.
9. Weinstock LB, Jasion VS. Serum-Derived Bovine Immunoglobulin/Protein Isolate Therapy for Patients with Refractory Irritable Bowel Syndrome. Open Journal of Gastroenterology. 2014;4:329-34.
10. Shafran I, Burgunder P, Wei D, Young HE, Klein G, Burnett BP. Management of inflammatory bowel disease with oral serum-derived bovine immunoglobulin. Therapeutic Advances in Gastroenterology. 2015;8(6):331-9. PubMed PMID: 26557889. Pubmed Central PMCID: PMC4622288. doi: 10.1177/1756283X15593693
11. Horgan A, Maas KJ, Henderson A, Detzel CJ, Weaver EM. Serum-derived bovine immunoglobulin/protein isolate binds to pathogen-associated molecular patterns. FASEB Journal. 2014; 128: Suppl No. 1; 836.6.
12. Weaver EM, Klein GL, DeVries BK, Maas KJ, Shaw AL. Endotoxin Neutralization activity (ENA) of bovine plasma and bovine Immunoglobulin (IgG)-rich fractions as compared to human plasma. FASEB Journal. 2013;27:1079.58.
13. Detzel CJ, Horgan A, Henderson AL, Petschow BW, Warner CD, Maas KJ, Weaver EM. Bovine immunoglobulin/protein isolate binds pro-inflammatory bacterial compounds and prevents immune activation in an intestinal co-culture model. PloS One. 2015;10(4):e0120278. PubMed PMID: 25830826. Pubmed Central PMCID: PMC4382133. doi: 10.1371/journal.pone.0120278
14. Petschow BW, Blikslager AT, Weaver EM, Campbell JM, Polo J, Shaw AL, Burnett BP, Klein GL, Rhoads JM. Bovine immunoglobulin protein isolates for the nutritional management of enteropathy. World Journal of Gastroenterology. 2014;20(33):11713-26. PubMed PMID: 25206275. Pubmed Central PMCID: 4155361. doi: 10.3748/wjg.v20.i33.11713
15. Asmuth DM, Ma ZM, Albanese A, Sandler NG, Devaraj S, Knight TH, Flynn NM, Yotter T, Garcia JC, Tsuchida E, Wu TT, Douek DC, Miller CJ. Oral Serum-Derived Bovine Immunoglobulin Improves Duodenal Immune Reconstitution and Absorption Function in Patients with HIV Enteropathy. AIDS. 2013;27:2207-17. PubMed PMID: 23660579. Pubmed Central PMCID: 3754419. doi: 10.1097/QAD.0b013e328362e54c
16. Asmuth DM, Hinkle JE, LaMarca A, Fichtenbaum CJ, Somsouk M, Utay NS, Shaw AL, Petschow BW, Detzel CJ, Weaver EM. Evaluation of oral serum-derived bovine immunoglobulins in HIV-infected patients with chronic idiopathic diarrhea. HIV Clinical Trials. 2017;18(5-6):205-13. PubMed PMID: 29210625. doi: 10.1080/15284336.2017.1401256
17. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-6. PubMed PMID: 2081237.
18. Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JF. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36(7):529-35. PubMed PMID: 14563446.
19. Fasano A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiological Reviews. 2011;91:151-75. doi: 10.1152/physrev.00003.2008
20. Perez-Bosque A, Miro L, Maijo M, Polo J, Campbell JM, Russell L, Crenshaw JD, Weaver E, Moreto M. Oral Serum-Derived Bovine Immunoglobulin/Protein Isolate Has Immunomodulatory Effects on the Colon of Mice that Spontaneously Develop Colitis. PLoS One. 2016;11(5):e0154823. PubMed PMID: 27139220. Pubmed Central PMCID: Pmc4854409. doi: 10.1371/journal.pone.0154823
21. Valentin N, Camilleri M, Carlson P, Harrington SC, Eckert D, O'Neill J, Burton D, Chen J, Shaw AL, Acosta A. Potential mechanisms of effects of serum-derived bovine immunoglobulin/protein isolate therapy in patients with diarrhea-predominant irritable bowel syndrome. Physiol Rep. 2017;5(5). PubMed PMID: 28275113. Pubmed Central PMCID: Pmc5350178. doi: 10.14814/phy2.13170
22. Ananthakrishnan AN, Luo C, Yajnik V, Khalili H, Garber JJ, Stevens BW, Cleland T, Xavier RJ. Gut Microbiome Function Predicts Response to Anti-integrin Biologic Therapy in Inflammatory Bowel Diseases. Cell Host Microbe. 2017;21(5):603-10.e3. PubMed PMID: 28494241. Pubmed Central PMCID: Pmc5705050. doi: 10.1016/j.chom.2017.04.010
23. Li Y, Ostergaard MV, Jiang P, Chatterton DE, Thymann T, Kvistgaard AS, Sangild PT. Whey protein processing influences formula-induced gut maturation in preterm pigs. J Nutr. 2013;143(12):1934-42. PubMed PMID: 24047702. doi: 10.3945/jn.113.182931
24. Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10(10):1096-100. PubMed PMID: 22902773. Pubmed Central PMCID: Pmc3458511. doi: 10.1016/j.cgh.2012.08.012
25. El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123(5):1607-15. PubMed PMID: 12404235.
26. Mokkala K, Roytio H, Munukka E, Pietila S, Ekblad U, Ronnemaa T, Eerola E, Laiho A, Laitinen K. Gut Microbiota Richness and Composition and Dietary Intake of Overweight Pregnant Women Are Related to Serum Zonulin Concentration, a Marker for Intestinal Permeability. J Nutr. 2016;146(9):1694-700. PubMed PMID: 27466607. doi: 10.3945/jn.116.235358
27. Malickova K, Francova I, Lukas M, Kolar M, Kralikova E, Bortlik M, Duricova D, Stepankova L, Zvolska K, Pankova A, Zima T. Fecal zonulin is elevated in Crohn's disease and in cigarette smokers. Pract Lab Med. 2017;9:39-44. PubMed PMID: 29034305. Pubmed Central PMCID: Pmc5633835. doi: 10.1016/j.plabm.2017.09.001
28. Sarker P, Banik A, Stromberg R, Gudmundsson GH, Raqib R, Agerberth B. Treatment with Entinostat Heals Experimental Cholera by Affecting Physical and Chemical Barrier Functions of Intestinal Epithelia. Antimicrob Agents Chemother. 2017;61(7). PubMed PMID: 28438947. Pubmed Central PMCID: Pmc5487680. doi: 10.1128/aac.02570-16
29. Sereti I, Krebs SJ, Phanuphak N, Fletcher J, Slike B, Pinyakorn S, O'Connell R, Rupert A, Chomont N, Valcour V, Kim J, Robb M, Michael N, Douek D, Ananworanich J, Utay N. Initiation of antiretroviral therapy in early HIV infection reduces but does not abrogate chronic residual inflammation. Clin Infect Dis. 2016:Accepted for publication.
30. Hatano H, Hayes TL, Dahl V, Sinclair E, Lee TH, Hoh R, Lampiris H, Hunt PW, Palmer S, McCune JM, Martin JN, Busch MP, Shacklett BL, Deeks SG. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis. 2011;203(7):960-8. PubMed PMID: 21402547. Pubmed Central PMCID: PMC3068029. doi: 10.1093/infdis/jiq138
31. Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, McLaughlin B, Landay AL, Adeyemi O, Gilman LE, Clagett B, Rodriguez B, Martin JN, Schacker TW, Shacklett BL, Palmer S, Lederman MM, Deeks SG. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood. 2013;121(23):4635-46. PubMed PMID: 23589670. Pubmed Central PMCID: PMC3685899. doi: 10.1182/blood-2012-06-436345
32. Castillo-Mancilla JR, Phillips AN, Neaton JD, Neuhaus J, Collins S, Mannheimer S, Pett S, Touzeau-Romer V, Polizzotto MN, Lundgren JD, Gardner EM, Group ISS. Association of Suboptimal Antiretroviral Therapy Adherence With Inflammation in Virologically Suppressed Individuals Enrolled in the SMART Study. Open Forum Infect Dis. 2018;5(1):ofx275. PubMed PMID: 29362724. Pubmed Central PMCID: PMC5772402. doi: 10.1093/ofid/ofx275
33. Hsu DC, Ma YF, Hur S, Li D, Rupert A, Scherzer R, Kalapus SC, Deeks S, Sereti I, Hsue PY. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive antiretroviral therapy. Aids. 2016;30(13):2065-74. PubMed PMID: 27177313. Pubmed Central PMCID: Pmc5586221. doi: 10.1097/qad.0000000000001149
34. Nordell AD, McKenna M, Borges AH, Duprez D, Neuhaus J, Neaton JD. Severity of cardiovascular disease outcomes among patients with HIV is related to markers of inflammation and coagulation. J Am Heart Assoc. 2014;3(3):e000844. PubMed PMID: 24870935. Pubmed Central PMCID: Pmc4309077. doi: 10.1161/jaha.114.000844
35. Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, Skoutelis A, Goetz MB, Phillips AN. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200(6):973-83. PubMed PMID: 19678756. Pubmed Central PMCID: Pmc2892757. doi: 10.1086/605447
36. Grund B, Baker JV, Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, Cohen CJ, Phillips A, Lundgren JD, Neaton JD. Relevance of Interleukin-6 and D-Dimer for Serious Non-AIDS Morbidity and Death among HIV-Positive Adults on Suppressive Antiretroviral Therapy. PLoS One. 2016;11(5):e0155100. PubMed PMID: 27171281. Pubmed Central PMCID: Pmc4865234. doi: 10.1371/journal.pone.0155100
37. Wada NI, Bream JH, Martinez-Maza O, Macatangay B, Galvin SR, Margolick JB, Jacobson LP. Inflammatory Biomarkers and Mortality Risk Among HIV-Suppressed Men: A Multisite Prospective Cohort Study. Clin Infect Dis. 2016. PubMed PMID: 27343547. doi: 10.1093/cid/ciw409
38. Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Medicine. 2008;5(10):e203. PubMed PMID: 18942885.
39. Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, Gripshover B, Salata RA, Taege A, Lisgaris M, McComsey GA, Kirchner E, Baum J, Shive C, Asaad R, Kalayjian RC, Sieg SF, Rodriguez B. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. Journal of Infectious Diseases. 2011;204(8):1217-26. PubMed PMID: 21917895. Pubmed Central PMCID: Pmc3218674. doi: 10.1093/infdis/jir507
40. Kasang C, Kalluvya S, Majinge C, Kongola G, Mlewa M, Massawe I, Kabyemera R, Magambo K, Ulmer A, Klinker H, Gschmack E, Horn A, Koutsilieri E, Preiser W, Hofmann D, Hain J, Muller A, Dolken L, Weissbrich B, Rethwilm A, Stich A, Scheller C. Effects of Prednisolone on Disease Progression in Antiretroviral-Untreated HIV Infection: A 2-Year Randomized, Double-Blind Placebo-Controlled Clinical Trial. PLoS One. 2016;11(1):e0146678. PubMed PMID: 26812052. Pubmed Central PMCID: Pmc4727920. doi: 10.1371/journal.pone.0146678
41. Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117(21):5582-90. PubMed PMID: 21403129. doi: 10.1182/blood-2010-12-322453
42. Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, Mitsuyasu RT, Kilby JM. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. Journal of Clinical Investigation. 1999;103(10):1391-8. PubMed PMID: 10330421. Pubmed Central PMCID: PMC408455. doi: 10.1172/JCI5863
43. Benveniste O, Flahault A, Rollot F, Elbim C, Estaquier J, Pedron B, Duval X, Dereuddre-Bosquet N, Clayette P, Sterkers G, Simon A, Ameisen JC, Leport C. Mechanisms involved in the low-level regeneration of CD4+ cells in HIV-1-infected patients receiving highly active antiretroviral therapy who have prolonged undetectable plasma viral loads. Journal of Infectious Diseases. 2005;191(10):1670-9. PubMed PMID: 15838794. doi: 10.1086/429670
44. Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, Fine DM, Coombs RW, Jacobson JM, Landay AL, Douek DC, Tressler R, Read SW, Wilson CC, Deeks SG, Lederman MM, Gandhi RT. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210(10):1549-54. PubMed PMID: 24864123. Pubmed Central PMCID: Pmc4215074. doi: 10.1093/infdis/jiu305
45. Tenorio AR, Chan ES, Bosch RJ, Macatangay BJ, Read SW, Yesmin S, Taiwo B, Margolis DM, Jacobson JM, Landay AL, Wilson CC. Rifaximin has a marginal impact on microbial translocation, T-cell activation and inflammation in HIV-positive immune non-responders to antiretroviral therapy - ACTG A5286. J Infect Dis. 2015;211(5):780-90. PubMed PMID: 25214516. Pubmed Central PMCID: Pmc4334803. doi: 10.1093/infdis/jiu515
46. Somsouk M, Dunham RM, Cohen M, Albright R, Abdel-Mohsen M, Liegler T, Lifson J, Piatak M, Gorelick R, Huang Y, Wu Y, Hsue PY, Martin JN, Deeks SG, McCune JM, Hunt PW. The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PLoS One. 2014;9(12):e116306. PubMed PMID: 25545673. Pubmed Central PMCID: Pmc4283685. doi: 10.1371/journal.pone.0116306
47. Bandera A, De Benedetto I, Bozzi G, Gori A. Altered gut microbiome composition in HIV infection: causes, effects and potential intervention. Curr Opin HIV AIDS. 2018;13(1):73-80. PubMed PMID: 29045252. doi: 10.1097/coh.0000000000000429
48. Nixon DE, Bosch RJ, Chan ES, Funderburg NT, Hodder S, Lake JE, Lederman MM, Klingman KL, Aberg JA. Effects of atorvastatin on biomarkers of immune activation, inflammation, and lipids in virologically suppressed, human immunodeficiency virus-1-infected individuals with low-density lipoprotein cholesterol < 130 mg/dL (AIDS Clinical Trials Group Study A5275). J Clin Lipidol. 2017;11(1):61-9. PubMed PMID: 28391912. Pubmed Central PMCID: Pmc5407297. doi: 10.1016/j.jacl.2016.09.017
49. Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, Bandera A, Capetti A, Rizzardini G, Clerici M. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. Aids. 2010;24(13):1991-2000. PubMed PMID: 20651586. doi: 10.1097/QAD.0b013e32833c93ce
50. McComsey GA, Whalen CC, Mawhorter SD, Asaad R, Valdez H, Patki AH, Klaumunzner J, Gopalakrishna KV, Calabrese LH, Lederman MM. Placebo-controlled trial of prednisone in advanced HIV-1 infection. Aids. 2001;15(3):321-7. PubMed PMID: 11273211.
51. Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, Clagett B, Robinson J, Lederman MM, McComsey GA. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396-404. PubMed PMID: 25514794. Pubmed Central PMCID: Pmc4334694. doi: 10.1097/qai.0000000000000478
52. Hsue P, Deeks SG, Ishai AE, Hur S, Li D, Sterman F, Lalezari J, Rupert A, Ganz P, Tawakol A. IL-1β inhibition significantly reduces atherosclerotic inflammation in treated HIV. Conference on Retroviruses and Opportunistic Infections. 2017:126.
53. Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. Journal of Leukocyte Biology. 2010;87(6):1001-9. PubMed PMID: 20200405. Pubmed Central PMCID: PMC4057658. doi: 10.1189/jlb.0809542
54. Gutierrez F, Padilla S, Masia M, Iribarren JA, Moreno S, Viciana P, Hernandez-Quero J, Aleman R, Vidal F, Salavert M, Blanco JR, Leal M, Dronda F, Perez Hoyos S, del Amo J, Co RM. Patients' characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Curr HIV Res. 2008;6(2):100-7. PubMed PMID: 18336257.
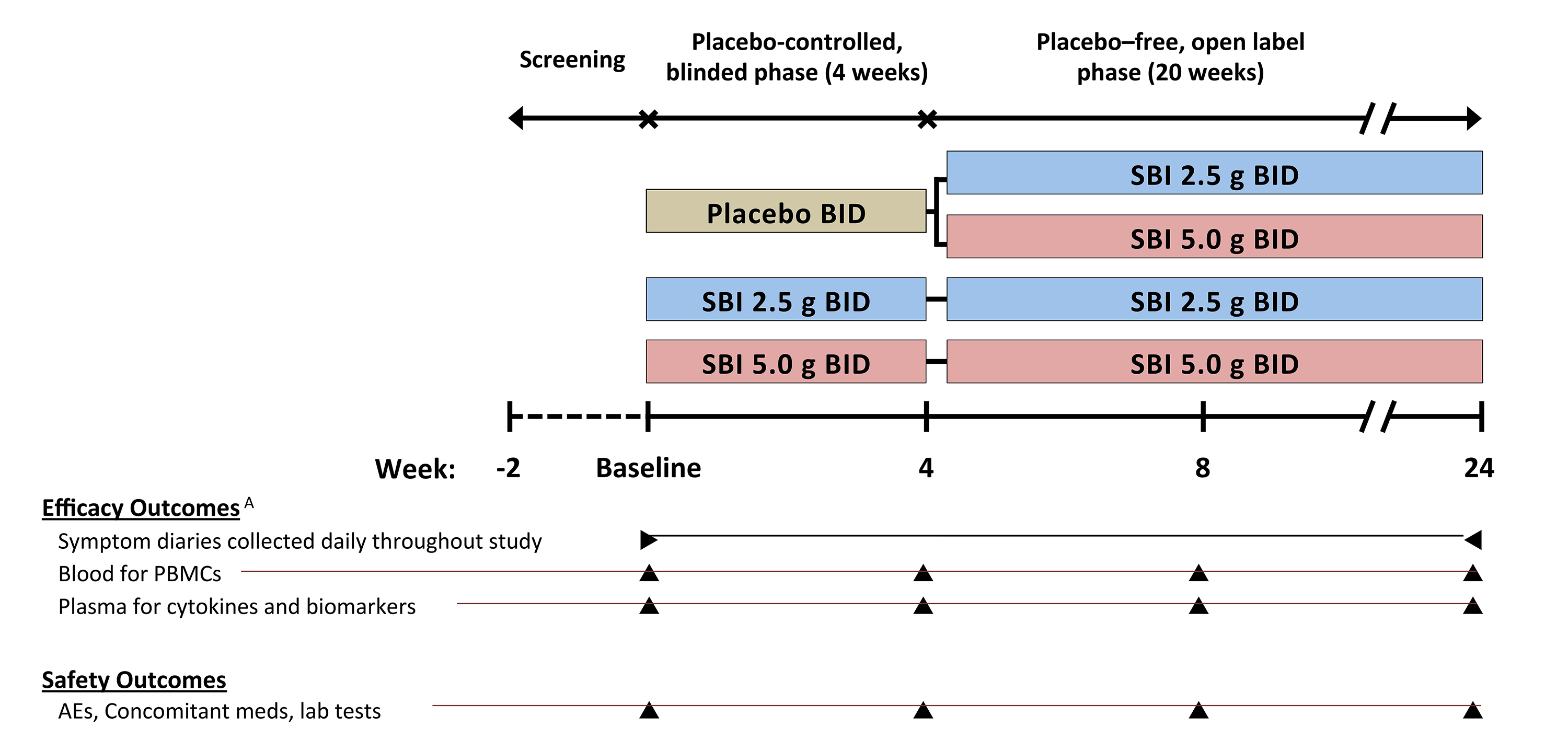
Supplementary Figure 1. Study design and schedule of outcome procedures.
Supplementary Table 1. Within-Group Comparisons for Randomized Treatment Groupsa at Week 4
|
Variable |
Treatment
|
n Median (Q1-Q3) |
P-valueb |
|||||
|
Baseline |
Week 4 |
Delta |
||||||
|
CD4 (cells/uL) |
Placebo |
35 |
523 (397–921) |
35 |
635 (330–858) |
34 |
-12.50 (-133–58) |
0.396 |
|
SBI 2.5 g |
34 |
813 (469–939) |
33 |
730 (483–1073) |
33 |
0 (-96–119) |
0.752 |
|
|
SBI 5.0 g |
33 |
672 (478–776) |
31 |
597 (423–723) |
31 |
-42 (-115–58) |
0.109 |
|
|
|
||||||||
|
CD4/CD8 |
Placebo |
35 |
0.69 (0.46–1.22) |
35 |
0.76 (0.50–1.17) |
34 |
0 (-0.06–0.07) |
0.667 |
|
SBI 2.5 g |
34 |
0.76 (0.42–1.22) |
33 |
0.80 (0.47–1.29) |
33 |
0 (-0.05–0.04) |
0.992 |
|
|
SBI 5.0 g |
33 |
0.68 (0.53–1) |
31 |
0.73 (0.48–0.89) |
31 |
0 (-0.06–0.07) |
0.880 |
|
|
|
||||||||
|
I-FABP (ng/mL) |
Placebo |
35 |
1.94 (1.10–3.32) |
35 |
1.74 (1.12–3.13) |
34 |
0 (-0.76–0.68) |
0.910 |
|
SBI 2.5 g |
34 |
1.69 (0.99–2.69) |
31 |
1.54 (1.03–2.12) |
31 |
-0.11 (-0.75–0.55) |
0.592 |
|
|
SBI 5.0 g |
33 |
1.81 (1.12–2.66) |
31 |
2.07 (1.19–2.68) |
31 |
0.30 (-1.08–1.07) |
0.674 |
|
|
|
||||||||
|
Zonulin (ng/mL) |
Placebo |
35 |
34.76 (28.39–44.63) |
35 |
36.28 (24.48–51.49) |
34 |
-3.06 (-11.14–10.91) |
0.913 |
|
SBI 2.5 g |
34 |
33.65 (18.89–41.77) |
31 |
34.84 (25.23–46.11) |
31 |
3.28 (-13.42–14.13) |
0.954 |
|
|
SBI 5.0 g |
33 |
36.07 (22.87–49.35) |
31 |
26.54 (20.37–45.94) |
31 |
-5.90 (-14.69–9.21) |
0.335 |
|
|
|
||||||||
|
Flagellin (ng/mL) |
Placebo |
35 |
5.01 (3.87–8.63) |
33 |
4.79 (2.28–7.95) |
32 |
-0.09 (-2.42–2.49) |
> 0.999 |
|
SBI 2.5 g |
34 |
4.06 (2.21–5.98) |
31 |
5.15 (2.62–7.20) |
31 |
0 (-1.42–2.74) |
0.533 |
|
|
SBI 5.0 g |
33 |
4.93 (2.97–7.42) |
29 |
4.91 (2.56–7.24) |
29 |
-0.11 (-2.37–0.76) |
0.360 |
|
|
|
||||||||
|
sCD14 (ng/mL) |
Placebo |
35 |
1.84 (1.59–2.04) |
35 |
1.88 (1.52–2.24) |
34 |
-0.03 (-0.19–0.22) |
0.848 |
|
SBI 2.5 g |
34 |
2.12 (1.70–2.28) |
31 |
1.95 (1.65–2.30) |
31 |
-0.13 (-0.38–0.26) |
0.340 |
|
|
SBI 5.0 g |
33 |
1.89 (1.48–2.23) |
31 |
1.81 (1.49–2.22) |
31 |
0.02 (-0.22–0.26) |
0.554 |
|
|
|
||||||||
|
IL-6 (pg/mL) |
Placebo |
35 |
1.29 (0.91–2.82) |
35 |
1.25 (0.86–1.83) |
34 |
-0.23 (-1.69–0.47) |
0.045 |
|
SBI 2.5 g |
34 |
1.79 (1.31–2.71) |
31 |
1.37 (0.82–1.83) |
31 |
-0.46 (-1.09–0.49) |
0.194 |
|
|
SBI 5.0 g |
33 |
1.45 (0.91–2.36) |
31 |
1.20 (0.32–2.30) |
31 |
-0.25 (-0.53–0.70) |
0.776 |
|
a Analysis population = Participants randomized to LD-SBI, HD-SBI or PBO at initiation of study
b Wilcoxon Signed-Rank test
Abbreviations: SBI, Serum-derived bovine immunoglobulin/protein isolate; I-FABP, intestinal fatty acid binding protein; sCD14, soluble CD14