Christopher G. Hauck1, Pearlie P. Chong1, Melissa B. Miller2, Katarzyna Jamieson3, Jason P. Fine4, Matthew C. Foster3, Thomas C. Shea3, David van Duin1
Preliminary results from this study were presented at IDWeek; October 7-11, 2015, San Diego, CA.
Fluoroquinolone (FQ) antibiotics have been shown to reduce mortality and the number of febrile episodes when used as prophylaxis during neutropenia. Prior studies suggest that prophylaxis may result in increasing rates of FQ resistance. Fluoroquinolone non-susceptibility trends in Escherichia coli isolated from blood and urine cultures were evaluated over a 16-year period during which prophylaxis was initiated in patients with hematologic malignancies and stem cell transplants. Non-susceptibility rates increased after the introduction of prophylaxis, with yearly non-susceptibility rates rising from 30%–33% to 40%–88% in blood isolates. The high rates of non-susceptibility now observed raise concerns about the continued efficacy of FQ prophylaxis. This concern exists particularly in those patients undergoing stem cell transplants where the total FQ non-susceptibility rates over the study period were 82.3%. Further evaluation of the effect of FQ prophylaxis on antibiotic resistance and its efficacy in the setting of increased rates of resistance is warranted.
Keywords: Fluoroquinolone resistance, Escherichia coli, stem cell transplant, hematologic malignancy
E. coli Quinolone Resistance in Hematologic Malignancy
Neutropenia and the associated risk of infection is a consistent problem in the treatment of leukemia and lymphoma. Studies have shown that 12%-43% of patients undergoing treatment for leukemia have an episode of bacteremia during the course of their care [1-7]. Guidelines for the management of neutropenia in both hematologic malignancy (HM) patients and hematopoietic stem cell transplant (HSCT) recipients recommend fluoroquinolone (FQ) prophylaxis based on studies showing a significant reduction in the number of documented infections and episodes of bacteremia [7-10]. Importantly, the Infectious Disease Society of America (IDSA) neutropenia in cancer guidelines also recommend having a strategy to monitor rates of FQ resistance among gram-negative bacilli.
In this retrospective study, the primary goal was to evaluate the trend of FQ non-susceptibility rates in E. coli isolates from patients’ blood and urine cultures at our institution from 2000 to 2015. FQ non-susceptibility rates in isolates obtained from an initial blood or urine culture from patients with HM/HSCT were compared to non-HM/HSCT patients.
Between 2005 and 2015, HSCT recipients and high risk patients with HM who were neutropenic (absolute neutrophil count (ANC) < 500 cells/mm3) received universal FQ prophylaxis (levofloxacin 500mg PO daily) per institutional protocol. Prior to 2005, use of FQ prophylaxis was at the discretion of the treating physician. Patients with HM are considered high risk if anticipated duration of neutropenia > 7 days and/or anticipated ANC < 100 cells/mm3. FQ prophylaxis was discontinued when the ANC was > 500 cells/mm3. The study was performed under a waiver of informed consent. The study was approved by the IRB of the University of North Carolina (IRB 14-0842).
All E. coli isolates were tested for susceptibility to ciprofloxacin, levofloxacin, or both. Isolates were defined as non-susceptible if testing determined them to be either intermediate or resistant to fluoroquinolones using standardized testing and interpretive criteria as published by the Clinical and Laboratory Standards Institute. Susceptibility testing from 2000 to mid-2009 was performed using Kirby-Bauer disk diffusion. After June of 2009 susceptibility testing was performed using VITEK2 (bioMerieux, Durham, NC).
The percentage of cultures showing FQ non-susceptibility per year was calculated. Each patient was included in the evaluation only once based on their first culture positive for E. coli (blood or urine). Comparisons between HM/HSCT and non-HM/HSCT patients were performed with Fisher’s exact or Pearson’s chi-square test.
For the blood culture results, an interrupted time series analysis was performed with the objective of assessing differences in the trend in rates of FQ resistance before and after instituting a protocol of antibiotic prophylaxis for neutropenia. The HM/HSCT and non-HM/HSCT cohorts were analyzed separately using the linear regression function ‘lm’ in the software package R, which assumes independent errors. The interrupted time series model was performed using time and preprotocolized vs postprotocolized antibiotic prophylaxis as the covariates. No significant time effects were seen in the analyses, possibly due to small sample size.
A total of 1,324 non-HM/HSCT patients and 149 HM/HSCT (HM n = 98, HSCT n = 51) patients with blood cultures positive for E. coli were identified from 2000 to 2015. From 2000 to 2013 a total of 23,382 non-HM/HSCT patients and 242 HM/HSCT (HM n = 210, HSCT n = 57) patients were identified with E. coli positive urine cultures. The underlying malignancy diagnoses in the HM/HSCT patients are described in Table 1.
Table 1. Primary hematologic malignancy diagnosis of patients
|
Underlying Malignancy |
All patients n = 355 |
HM Blood n = 98 |
SCT Blood n = 51 |
HM Urine n = 187 |
SCT Urine n = 54 |
|
Acute leukemia |
116 (32) |
46 (47) |
23 (49) |
45 (24) |
16 (30) |
|
|
40 |
12 |
5 |
23 |
3 |
|
|
76 |
34 |
18 |
23 |
13 |
|
Chronic Lymphocytic Leukemia |
31 (9) |
7 (7) |
- |
26 (14) |
- |
|
Chronic Myelogenous Leukemia |
16 (5) |
3 (3) |
2 (3) |
8 (4) |
4 (7) |
|
Hodgkin Lymphoma |
13 (4) |
2 (2) |
1 (3) |
9 (5) |
2 (4) |
|
Non-Hodgkin Lymphoma |
126 (35) |
33 (34) |
9 (17) |
84 (45) |
12 (22) |
|
|
46 |
13 |
1 |
34 |
4 |
|
|
33 |
6 |
4 |
20 |
5 |
|
|
10 |
1 |
- |
10 |
- |
|
|
7 |
- |
- |
6 |
1 |
|
|
9 |
6 |
1 |
3 |
- |
|
|
21 |
7 |
3 |
11 |
2 |
|
Myelodysplastic disorders |
30 (8) |
7 (7) |
8 (14) |
15 (8) |
3 (6) |
|
Multiple Myeloma |
23 (6) |
- |
8 (14) |
- |
17 (32) |
|
Data presented as n (%) with percent representing proportion within the column it occurs Abbreviations: HM, hematologic malignancy; SCT, stem cell transplantation |
|||||
The FQ non-susceptible rates in E. coli isolates in blood cultures were 81 out of 149 (54%) in the HM/HSCT group and 462/1,445 (32%) in the non-HM/HSCT group (P < 0.001). Within the HM/HSCT group, HSCT patients were more likely to have bacteremia caused by E. coli non-susceptible to FQ, compared to HM patients; 42 of 51 (82.3%) vs 39 of 98 (39.8%), P < 0.001.
In urine isolates the FQ non-susceptibility rates for E. coli isolates were 76 of 267, (28%) in the HM/HSCT group and 3,408 of 23,382 (14.6%) in the non-HM/HSCT group (P < 0.001). Similar to the results from E. coli blood isolates, E. coli from HSCT patients displayed a higher rate of resistance (27 of 57, 47%), as compared to HM patients (49 of 210, 23%, P < 0.001).
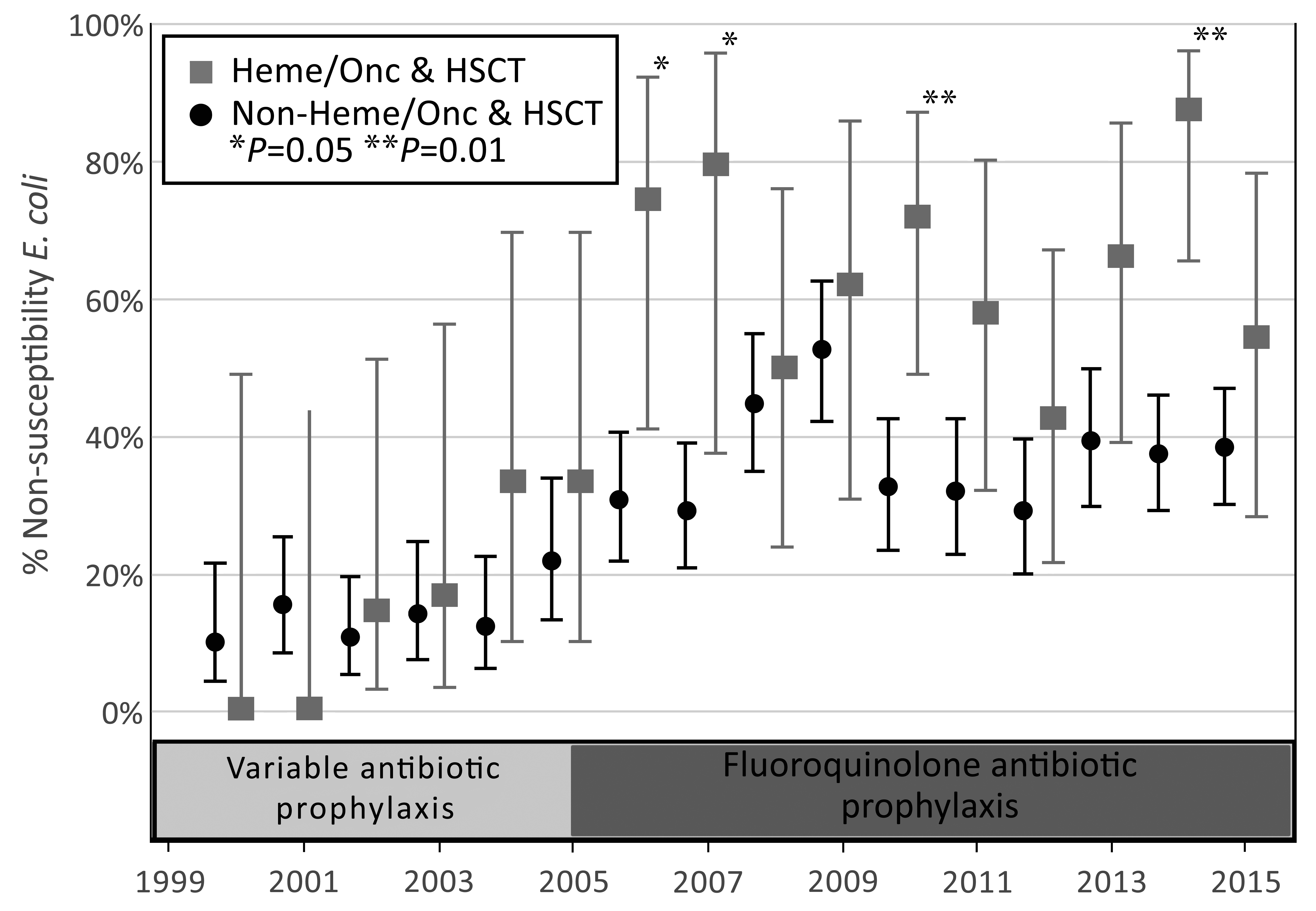
The annual rate of FQ non-susceptibility in E. coli increased across the study period of 2000-2015 for blood isolates, and the annual rate of FQ non-susceptibility in E. coli increased across the study for both blood and urine isolates (Figures 1 and 2). Starting in 2004 the numbers of E. coli that were FQ non-susceptible began to increase at a greater rate in the HM/HSCT group compared to the control group in both blood and urine culture groups. The total rate of non-susceptible E. coli isolates from the first positive blood cultures in the HM/HSCT group after the initiation of protocolized use of fluoroquinolone prophylaxis (2005-2015) was 63.6%, compared to 38.0% in the control group (P < 0.001). Over the period of 2000 to 2013 the rate of non-susceptible E. coli isolates from the first positive urine cultures was 33.5% in HM/HSCT in contrast to 18.3% in the control group (P < 0.001). Additionally, the absolute number of FQ non-susceptible E. coli isolates from the first blood cultures in the HM/HSCT group increased from an average of 0.8 cases/year prior to 2005, to 7.0 cases/year from 2005-2015. The average number of FQ non-susceptible E. coli isolates from the first urine cultures showed a similar trend with 1.7 cases/year from 2000-2004 and 6.7 cases/year from 2005-2013.

Figure 1. Trend in fluoroquinolone non-susceptibility rates in first E. coli positive blood culture by year of occurrence, 2000-2015. The error bars represent 95% confidence intervals. HSCT, hematopoietic stem cell transplant.
Abbreviations: HSCT, hematopoietic stem cell transplant.
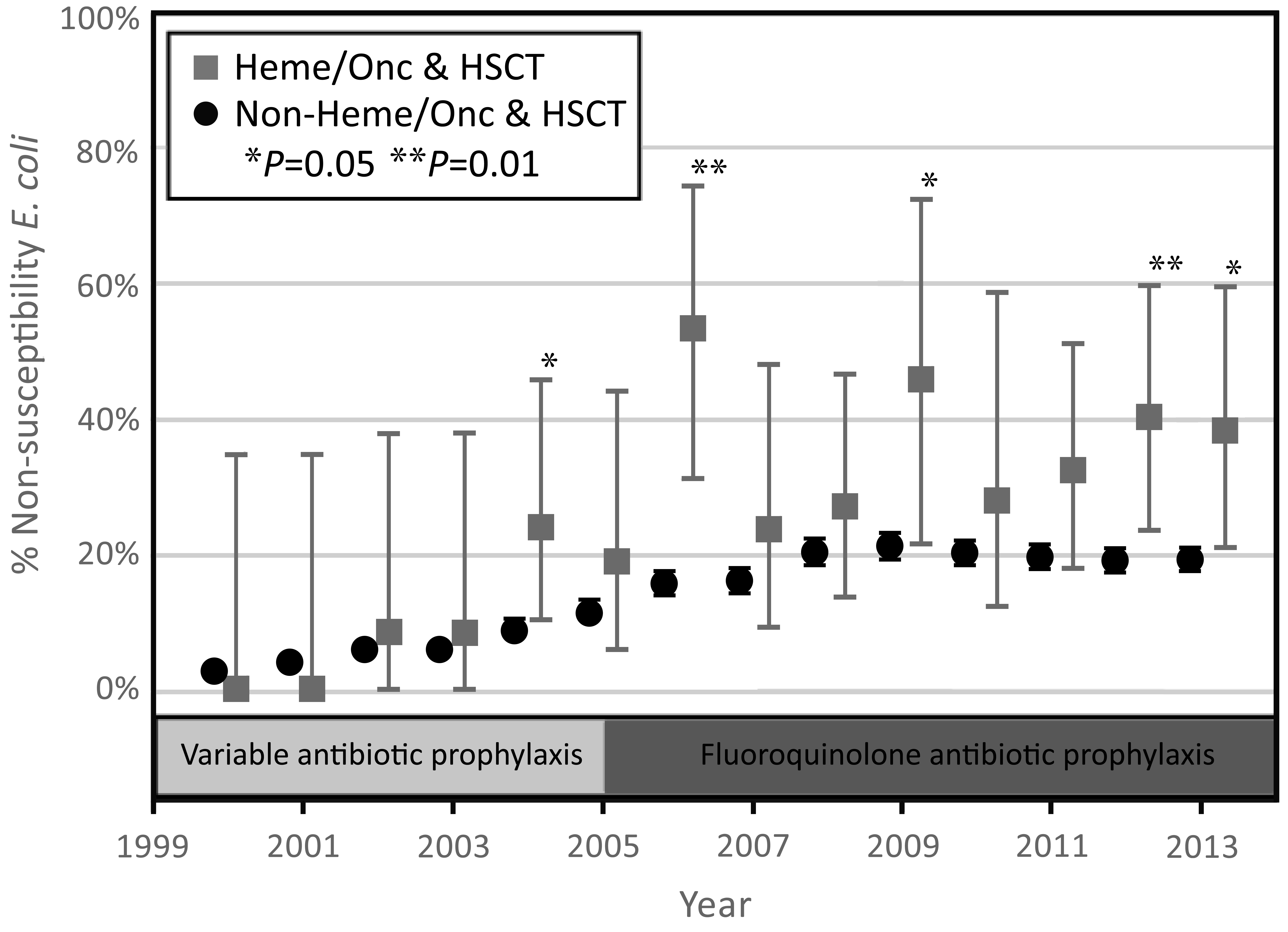
Figure 2. Trend in fluoroquinolone non-susceptibility rates in the first E. coli positive urine culture by year of occurrence, 2000-2013. The error bars represent 95% confidence intervals.
Abbreviations: HSCT, hematopoietic stem cell transplant.
An interrupted time series analysis was used to compare trends in FQ resistance before and after the initiation of protocolized antibiotic prophylaxis in 2005. Linear regression analyses were performed with the inclusion of the covariate of time. Comparison of the trends in non-susceptibility rates prior to 2005 for HM/HSCT and non-HM/HSCT were not statistically significant (P = 0.23). A comparison of the trend between the two groups after 2005 was just outside the margin of statistical significance (P = 0.06). Within groups, a comparison of before vs after 2005 was significant in the HM/HSCT group (P = 0.003) and not significant for the non-HM/HSCT group (P = 0.10), indicating that the only significant change in the trend of antibiotic resistance rates occurred in the HM/HSCT group after initiation of antimicrobial prophylaxis.
This trend of increasing FQ non-susceptibility in the HM/HSCT group presents questions about the efficacy of FQ antibiotics for neutropenic prophylaxis. The marked increase in E. coli non-susceptible to FQ from 2005 to 2007 in the HM/HSCT group is of particular concern because prophylaxis with levofloxacin became part of the standard neutropenia protocol in 2005. In prior studies looking at rates of FQ non-susceptibility after the introduction of prophylaxis, similar increases in non-susceptibility were seen in some [11-14], while others found no change in rates of non-susceptibility after the onset of prophylaxis [15-17]. The rise in resistance seen in this study is most likely an effect of increased FQ exposure, with the more significant rise seen in the HM/HSCT group likely reflecting the initiation of FQ prophylaxis. The observed highest rates of resistance in HSCT patients probably reflects increased FQ exposure, as these patients generally have had multiple neutropenic episodes.
Another concern pertains to the efficacy of prophylaxis in the setting of high baseline rates of FQ resistance. It is unknown whether the evidence for the benefit of prophylaxis continues to hold true in the setting where non-susceptibility rates are 40%-60%. This uncertainty exists particularly in the HSCT population where 82.3% of E. coli isolates were non-susceptible to FQ. However, these findings may reflect successful suppression of FQ-susceptible isolates, such that only FQ non-susceptible organisms cause breakthrough infections.
In conclusion, FQ non-susceptibility rates in E. coli isolated from blood and urine cultures increased over the study period, especially in
patients with hematologic malignancies and HSCT patients. This increase in rates of FQ non-susceptibility coincided with the implementation of levofloxacin
prophylaxis for patients with neutropenia at our institution. Whether this is a reversible trend remains to be determined.
1. Poutsiaka DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream infection after hematopoietic stem cell transplantation is associated with increased mortality. Bone Marrow Transplant. 2007;40(1):63-70. PubMed PMID: 216653881; 17468772. doi: 10.1038/sj.bmt.1705690
2. Mikulska M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, di Grazia C, Frassoni F, Bacigalupo A, Viscoli C. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant. 2009;15(1):47-53. PubMed PMID: 19135942. doi: 10.1016/j.bbmt.2008.10.024
3. Almyroudis NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, Kiehn TE, Pamer E, Papanicolaou GA. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7(1):11-7. doi: 10.1111/j.1399-3062.2005.00088.x
4. Busca A, Cavecchia I, Locatelli F, D'Ardia S, De Rosa FG, Marmont F, Ciccone G, Baldi I, Serra R, Gaido E, Falda M. Blood stream infections after allogeneic stem cell transplantation: a single-center experience with the use of levofloxacin prophylaxis. Transpl Infect Dis. 2012;14(1):40-8. PubMed PMID: 21599817. doi: 10.1111/j.1399-3062.2011.00650.x
5. Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, Incidence, and Susceptibility of Bacterial Bloodstream Isolates from 519 Bone Marrow Transplant Patients. Clin Infect Dis. 2001;33(7):947-53. doi: 10.1086/322604
6. Ninin E, Milpied N, Moreau P, André-Richet B, Morineau N, Mahé B, Vigier M, Imbert B-M, Morin O, Harousseau J-L, Richet H. Longitudinal Study of Bacterial, Viral, and Fungal Infections in Adult Recipients of Bone Marrow Transplants. Clin Infect Dis. 2001;33(1):41-7. doi: 10.1086/320871
7. Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, Allione B, D'Antonio D, Buelli M, Nosari AM, Cilloni D, Zuffa E, Cantaffa R, Specchia G, Amadori S, Fabbiano F, Deliliers GL, Lauria F, Foa R, Del Favero A. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353(10):977-87. PubMed PMID: 16148283. doi: 10.1056/NEJMoa044097
8. Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142(12 Pt 1):979-95. PubMed PMID: 15968013.
9. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young J-AH, Wingard JR. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi: 10.1093/cid/cir073
10. Recommendations of the Center for International Blood and Marrow Transplant Research (CIBMTR®) tNMDPN, the European Blood and Marrow Transplant Group (EBMT), the American Society of Blood and Marrow Transplantation (ASBMT), the Canadian Blood and Marrow Transplant Group (CBMTG), the Infectious Disease Society of America (IDSA), the Society for Healthcare Epidemiology of America (SHEA), the Association of Medical Microbiology and Infectious Diseases Canada (AMMI), and the Centers for Disease Control and Prevention (CDC), , Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143-238. PubMed PMID: 19747629. Pubmed Central PMCID: 3103296. doi: 10.1016/j.bbmt.2009.06.019
11. Bhusal Y, Mihu CN, Tarrand JJ, Rolston KV. Incidence of fluoroquinolone-resistant and extended-spectrum beta-lactamase-producing Escherichia coli at a comprehensive cancer center in the United States. Chemotherapy. 2011;57(4):335-8. PubMed PMID: 21912115. doi: 10.1159/000329661
12. Therriault BL, Wilson JW, Barreto JN, Estes LL. Characterization of bacterial infections in allogeneic hematopoietic stem cell transplant recipients who received prophylactic levofloxacin with either penicillin or doxycycline. Mayo Clin Proc. 2010;85(8):711-8. PubMed PMID: 20675508. Pubmed Central PMCID: 2912731. doi: 10.4065/mcp.2010.0006
13. De Rosa FG, Motta I, Audisio E, Frairia C, Busca A, Di Perri G, Marmont F. Epidemiology of bloodstream infections in patients with acute myeloid leukemia undergoing levofloxacin prophylaxis. BMC Infect Dis. 2013;13:563. PubMed PMID: 24289496. Pubmed Central PMCID: PMC4219399. doi: 10.1186/1471-2334-13-563
14. Macesic N, Morrissey CO, Cheng AC, Spencer A, Peleg AY. Changing microbial epidemiology in hematopoietic stem cell transplant recipients: increasing resistance over a 9-year period. Transpl Infect Dis. 2014;16(6):887-96. PubMed PMID: 25298044. doi: 10.1111/tid.12298
15. Miles-Jay A, Butler-Wu S, Rowhani-Rahbar A, Pergam SA. Incidence rate of fluoroquinolone-resistant gram-negative rod bacteremia among allogeneic hematopoietic cell transplantation patients during an era of levofloxacin prophylaxis. Biol Blood Marrow Transplant. 2015;21(3):539-45. PubMed PMID: 25498393. Pubmed Central PMCID: PMC4329069. doi: 10.1016/j.bbmt.2014.12.006
16. Chong Y, Yakushiji H, Ito Y, Kamimura T. Clinical impact of fluoroquinolone prophylaxis in neutropenic patients with hematological malignancies. Int J Infect Dis. 2011;15(4):e277-81. PubMed PMID: 21324723. doi: 10.1016/j.ijid.2010.12.010
17. Simondsen KA, Reed MP, Mably MS, Zhang Y, Longo WL. Retrospective analysis of fluoroquinolone prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation. J Oncol Pharm Pract. 2013;19(4):291-7. PubMed PMID: 23184539. doi: 10.1177/1078155212465215